The value of organs-on-chip for regulatory safety assessment
Main Article Content
Abstract
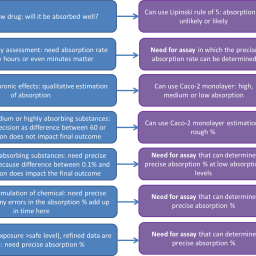
Organs-on-chip (OC) have gained much interest as animal-free toxicity testing methods due to their closer resemblance to human tissues and longer culture viability than conventional in vitro methods. The current paper discusses where and how OCs may take a role in the transition to a more predictive, animal-free safety assessment for regulatory purposes. From a preliminary analysis of a repeated dose toxicity database, ten organs of priority for OC development for regulatory use have been identified. For a number of these organs (lung, skin, liver, kidney, heart, and intestine), OCs are already at rather advanced stages of development, such that involvement of regulators becomes of value in the optimization towards fitness-for-purpose of these methods. For organs such as testis, spleen, brain, and stomach, OCs are much more premature, if existing at all. Therefore, developmental work on OCs for these latter organs is expected to stay in the academic arena for the coming time. A number of technical recommendations and some challenges to reaching final implementation are discussed. We recommend that the development of OCs goes forward together with the development of adverse outcome pathways (AOP) and that they are combined with other methods into integrated testing strategies. Overall, opportunities exist, but much still needs to be done. In our view, regular interactions in multi-stakeholder workshops on the application of animal-free innovations such as OCs will be beneficial.
Article Details
Articles are distributed under the terms of the Creative Commons Attribution 4.0 International license (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution and reproduction in any medium, provided the original work is appropriately cited (CC-BY). Copyright on any article in ALTEX is retained by the author(s).