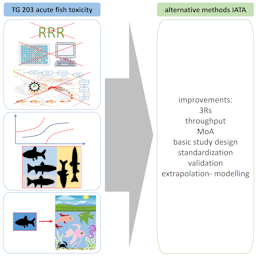
Limitations and uncertainties of acute fish toxicity assessments can be reduced using alternative methods
Main Article Content
Abstract
Information about acute fish toxicity is routinely required in many jurisdictions for environmental risk assessment of chemicals. This information is typically obtained using a 96-hour juvenile fish test for lethality according to OECD test guideline (TG) 203 or equivalent regional guidelines. However, TG 203 has never been validated using the criteria currently required for new test methods including alternative methods. Characterization of the practicality and validity of TG 203 is important to provide a benchmark for alternative methods. This contribution systematically summarizes the available knowledge on limitations and uncertainties of TG 203, based on methodological, statistical, and biological considerations. Uncertainties stem from the historic flexibility (e.g., use of a broad range of species) and constraints of the basic test design (e.g., no replication). Other sources of uncertainty arise from environmental safety extrapolation based on TG 203 data. Environmental extrapolation models, combined with data from alternative methods, including mechanistic indicators of toxicity, may provide at least the same level of environmental protection. Yet, most importantly, the 3R advantages of alternative methods allow a better standardization, characterization, and an improved basic study design. This can enhance data reliability and thus facilitate the comparison of chemical toxicity, as well as the environmental classifications and prediction of no-effect concentrations of chemicals. Combined with the 3R gains and the potential for higher throughput, a reliable assessment of more chemicals can be achieved, leading to improved environmental protection.
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.
Articles are distributed under the terms of the Creative Commons Attribution 4.0 International license (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution and reproduction in any medium, provided the original work is appropriately cited (CC-BY). Copyright on any article in ALTEX is retained by the author(s).
Adriaens, E., Barroso, J., Eskes, C. et al. (2014). Retrospective analysis of the Draize test for serious eye damage/eye irritation: Importance of understanding the in vivo endpoints under UN GHS/EU CLP for the development and evaluation of in vitro test methods. Arch Toxicol 88, 701-723. doi:10.1007/s00204-013-1156-8
Awkerman, J. A., Raimondo, S., Jackson, C. R. et al. (2014). Augmenting aquatic species sensitivity distributions with interspecies toxicity estimation models. Environ Toxicol Chem 33, 688-695. doi:10.1002/etc.2456
Barroso, J., Pfannenbecker, U., Adriaens, E. et al. (2017). Cosmetics Europe compilation of historical serious eye damage/eye irritation in vivo data analysed by drivers of classification to support the selection of chemicals for development and evaluation of alternative methods/strategies: The Draize eye test Reference Database (DRD). Arch Toxicol 91, 521-547. doi:10.1007/s00204-016-1679-x
Bauer, J. F., Thomas, P. C., Fouchard, S. Y. et al. (2018a). High-accuracy prediction of mechanisms of action using structural alerts. Comput Toxicol 7, 36-45. doi:10.1016/j.comtox.2018.06.004
Bauer, J. F., Thomas, P. C., Fouchard, S. Y. et al. (2018b). A new classification algorithm based on mechanisms of action. Comput Toxicol 5, 8-15. doi:10.1016/j.comtox.2017.11.001
Bejarano, A. C., Raimondo, S. and Barron, M. G. (2017). Framework for optimizing selection of interspecies correlation estimation models to address species diversity and toxicity gaps in an aquatic database. Environ Sci Technol 51, 8158-8165. doi:10.1021/acs.est.7b01493
Belanger, S. E., Rawlings, J. M. and Carr, G. J. (2013). Use of fish embryo toxicity tests for the prediction of acute fish toxicity to chemicals. Environ Toxicol Chem 32, 1768-1783. doi:10.1002/etc.2244
Belanger, S. E., Sanderson, H., Embry, M. R. et al. (2015). It is time to develop ecological thresholds of toxicological concern to assist environmental hazard assessment. Environ Toxicol Chem 34, 2864-2869. doi:10.1002/etc.3132
Benfenati, E., Manganaro, A. and Gini, G. (2013). VEGA-QSAR: AI inside a platform for predictive toxicology. CEUR Workshop Proceedings. http://ceur-ws.org/Vol-1107/
Braunbeck, T., Böhler, S., Knörr, S. et al. (2020). Development of an OECD Guidance Document for the Application of OECD Test Guideline 236 (Acute Fish Embryo Toxicity Test): The chorion structure and biotransformation capacities of zebrafish as boundary conditions for OECD Test Guideline 236 – German contributions to OECD project 2.54: Guidance Document on IATA for Fish Acute Toxicity Testing. UBA Texte 94/2020. https://bit.ly/345BHsc
Bundesgesetzblatt Bundesministerium für Umwelt, Naturschutz und Reaktorsicherheit (2005). Bekanntmachung der Neufassung des Abwasserabgabegesetzes. Teil I, Nr. 5, ausgegeben zu Bonn am 25 Januar 2005. http://extwprlegs1.fao.org/docs/pdf/ger35872b.pdf
Busquet, F., Strecker, R., Rawlings, J. M. et al. (2014). OECD validation study to assess intra- and inter-laboratory reproducibility of the zebrafish embryo toxicity test for acute aquatic toxicity testing. Regul Toxicol Pharmacol 69, 496-511. doi:10.1016/j.yrtph.2014.05.018
Carr, G. J., Bailer, A. J., Rawlings, J. M. et al. (2018). On the impact of sample size on median lethal concentration estimation in acute fish toxicity testing: Is n = 7/group enough? Environ Toxicol Chem 37, 1565-1578. doi:10.1002/etc.4098
Connors, K. A., Beasley, A., Barron, M. G. et al. (2019). Creation of a curated aquatic toxicology database: EnviroTox. Environ Toxicol Chem 38, 1062-1073. doi:10.1002/etc.4382
de-Vlaming, V. and Norberg-King, T. (1999). A review of single species toxicity tests: Are the tests reliable predictors of aquatic ecosystem community responses? EPA/600/R-97/114. https://bit.ly/3c1FI3T
Dimitrov, S., Detroyer, A., Piroird, C. et al. (2016). Accounting for data variability, a key factor in in vivo/in vitro relationships: Application to the skin sensitization potency (in vivo LLNA versus in vitro DPRA) example. J Appl Toxicol 36, 1568-1578. doi:10.1002/jat.3318
ECHA (2008). Guidance on Information Requirements and Chemical Safety Assessment. Chapter R.10: Characterisation of dose [concentration]-response for environment. Guidance for the implementation of REACH. https://bit.ly/37uWfg9
ECHA (2017a). Guidance on Information Requirements and Chemical Safety Assessment. Chapter R.11: PBT/vPvB assessment. Version 3.0. https://echa.europa.eu/guidance-documents/guidance-on-Information-requirements-and-chemical-safety-assessment
ECHA (2017b). Expert Workshop on the potential regulatory application of the Fish Embryo Acute Toxicity (FET) Test under REACH, CLP and the BPR, 3-4 May 2017 in Helsinki.
ECHA (2017c). Guidance on the Application of the CLP Criteria (Version 5.0). https://echa.europa.eu/guidance-documents/guidance-on-clp
EFSA (2014). Scientific opinion on good modelling practice in the context of mechanistic effect models for risk assessment of plant protection products. EFSA J 12, 3589. doi:10.2903/j.efsa.2014.3589
EFSA Scientific Committee, Benford, D., Halldorsson, T. et al. (2018). The principles and methods behind EFSA’s guidance on uncertainty analysis in scientific assessment. EFSA J 16, 235. doi:10.2903/j.efsa.2018.5122
European Commission (2020). Report on the statistics on the number of animals used for experimental and other scientific purposes in the member states of the European Union in 2015-2017. Commission staff working document. https://eur-lex.europa.eu/legal-content/EN/TXT/?qid=1581689520921&uri=CELEX:52020SC0010
Fischer, M., Belanger, S. E., Berckmans, P. et al. (2019). Repeatability and reproducibility of the RTgill-W1 cell line assay for predicting fish acute toxicity. Toxicol Sci 169, 353-364. doi:10.1093/toxsci/kfz057
Hahn, T., Diamond, J., Dobson, S. et al. (2014). Predicted no effect concentration derivation as a significant source of variability in environmental hazard assessments of chemicals in aquatic systems: An international analysis. Integr Environ Assess Manag 10, 30-36. doi:10.1002/ieam.1473
Helman, G., Shah, I., Williams, A. J. et al. (2019). Generalized read-across (GenRA): A workflow implemented into the EPA CompTox chemicals dashboard. ALTEX 36, 462-465. doi:10.14573/altex.1811292
Hoffmann, S., Kinsner-Ovaskainen, A., Prieto, P. et al. (2010). Acute oral toxicity: Variability, reliability, relevance and interspecies comparison of rodent LD50 data from literature surveyed for the ACuteTox project. Regul Toxicol Pharmacol 58, 395-407. doi:10.1016/j.yrtph.2010.08.004
Hoffmann, S., Kleinstreuer, N., Alépée, N. et al. (2018). Non-animal methods to predict skin sensitization (I): The Cosmetics Europe database. Crit Rev Toxicol 48, 344-358. doi:10.1080/10408444.2018.1429385
Hrovat, M., Segner, H. and Jeram, S. (2009). Variability of in vivo fish acute toxicity data. Regul Toxicol Pharmacol 54, 294-300. doi:10.1016/j.yrtph.2009.05.013
Hutchinson, T. H., Barrett, S., Buzby, M. et al. (2003). A strategy to reduce the numbers of fish used in acute ecotoxicity testing of pharmaceuticals. Environ Toxicol Chem 22, 3031-3036. doi:10.1897/02-558
IRGC (2017). Introduction to the IRGC Risk Governance Framework, revised version. International Risk Governance Center. doi:10.1007/978-1-4020-6799-0_6
ISO – International-Standard-Organization (1996a). Water quality – Determination of the acute lethal toxicity of substances to a freshwater fish [Brachydanio rerio Hamilton-Buchanan (Teleostei, Cyprinidae)] – Part 3: Flow-through method. ISO 7346-3:1996. doi:10.3403/00151595u
ISO (1996b). Water quality – Determination of the acute lethal toxicity of substances to a freshwater fish [Brachydanio rerio Hamilton-Buchanan (Teleostei, Cyprinidae)] – Part 2: Semi-static method. ISO 7346-2:1996. doi:10.3403/00151583u
ISO (1996c). Water quality – Determination of the acute lethal toxicity of substances to a freshwater fish [Brachydanio rerio Hamilton-Buchanan (Teleostei, Cyprinidae)] – Part 1: Static method. ISO 7346-1:1996. doi:10.3403/00151583u
ISO (2016). Water quality – Determination of the acute toxicity of waste water to zebrafish eggs (Danio rerio). ISO 15088:2007. doi:10.31030/1495364
ISO (2019). Water quality – Determination of acute toxicity of water samples and chemicals to a fish gill cell line (RTgill-W1). ISO 21115:2019. doi:10.3403/30361171u
Jeram, S., Sintes, J. M., Halder, M. et al. (2005). A strategy to reduce the use of fish in acute ecotoxicity testing of new chemical substances notified in the European Union. Regul Toxicol Pharmacol 42, 218-224. doi:10.1016/j.yrtph.2005.04.005
Kluver, N., Konig, M., Ortmann, J. et al. (2015). Fish embryo toxicity test: Identification of compounds with weak toxicity and analysis of behavioral effects to improve prediction of acute toxicity for neurotoxic compounds. Environ Sci Technol 49, 7002-7011. doi:10.1021/acs.est.5b01910
Knillmann, S., Stampfli, N. C., Beketov, M. A. et al. (2012). Intraspecific competition increases toxicant effects in outdoor pond microcosms. Ecotoxicology 21, 1857-1866. doi:10.1007/s10646-012-0919-y
Lammer, E., Carr, G. J., Wendler, K. et al. (2009). Is the fish embryo toxicity test (FET) with the zebrafish (Danio rerio) a potential alternative for the fish acute toxicity test? Comp Biochem Physiol C Toxicol Pharmacol 149, 196-209. doi:10.1016/j.cbpc.2008.11.006
Lemke, A. (1981). Interlaboratory Comparision: Acute Testing Set. US-EPA EPA-600/S3-81-005. https://nepis.epa.gov/Exe/ZyPURL.cgi?Dockey=2000TSC7.TXT
Leontaridou, M., Urbisch, D., Kolle, S. N. et al. (2017). The borderline range of toxicological methods: Quantification and implications for evaluating precision. ALTEX 34, 525-538. doi:10.14573/altex.1606271
Lillicrap, A., Moe, S. J., Wolf, R. et al. (2020). Evaluation of a Bayesian network for predicting acute fish toxicity from fish embryo toxicity data. Integr Environ Assess Manag 16, 452-460. doi:10.1002/ieam.4258
Low, Y., Sedykh, A., Fourches, D. et al. (2013). Integrative chemical-biological read-across approach for chemical hazard classification. Chem Res Toxicol 26, 1199-1208. doi:10.1021/tx400110f
Luechtefeld, T., Marsh, D., Rowlands, C. et al. (2018). Machine learning of toxicological big data enables read-across structure activity relationships (RASAR) outperforming animal test reproducibility. Toxicol Sci 165, 198-212. doi:10.1093/toxsci/kfy152
Maertens, A., Anastas, N., Spencer, P. J. et al. (2014). Green toxicology. ALTEX 31, 243-249. doi:10.14573/altex.1406181
Moe, S. J., Madsen, A. L., Connors, K. A. et al. (2020). Development of a hybrid Bayesian network model for predicting acute fish toxicity using multiple lines of evidence. Environ Modell Softw 126, 104655. doi:10.1016/j.envsoft.2020.104655
Mora, C., Tittensor, D. P., Adl, S. et al. (2011). How many species are there on Earth and in the ocean? PLoS Biol 9, e1001127. doi:10.1371/journal.pbio.1001127
NRC – National Research Council (2007). Toxicity Testing in the 21st Century: A Vision and a Strategy. Washington, DC, USA: The National Academies Press. doi:10.17226/11970
Natsch, A., Laue, H., Haupt, T. et al. (2018). Accurate prediction of acute fish toxicity of fragrance chemicals with the RTgill-W1 cell assay. Environ Toxicol Chem 37, 931-941. doi:10.1002/etc.4027
Norberg-King, T. J., Embry, M. R., Belanger, S. E. et al. (2018). An international perspective on the tools and concepts for effluent toxicity assessments in the context of animal alternatives: Reduction in vertebrate use. Environ Toxicol Chem 37, 2745-2757. doi:10.1002/etc.4259
OECD (2004). Test No. 202: Daphnia sp. Acute Immobilisation Test. OECD Guidelines for the Testing of Chemicals, Section 2. OECD Publishing, Paris. doi:10.1787/9789264069947-en
OECD (2007). Guidance on Grouping of Chemicals. OECD Series on Testing and Assessment, No. 80. OECD Publishing, Paris. https://bit.ly/37so20A
OECD (2010). Short Guidance on the Threshold Approach for Acute Fish Toxicity. OECD Series of Testing and Assessment, No. 126. OECD Publishing, Paris. https://bit.ly/3mrLsHF
OECD (2011a). Validation Report (Phase 1) for the Zebrafish Embryo Toxicity Test. OECD Series of Testing and Assessment, No. 157. OECD Publishing, Paris. http://www.oecd.org/env/ehs/testing/48572244.pdf
OECD (2011b). Test No. 201: Freshwater Alga and Cyanobacteria, Growth Inhibition Test. OECD Guidelines for the Testing of Chemicals, Section 2. OECD Publishing, Paris. doi:10.1787/9789264069923-en
OECD (2012a). Fish Testing Framework. OECD Series of Testing and Assessment, No. 171. OECD Publishing, Paris. https://bit.ly/3r37l3A
OECD (2012b). Test No. 305: Bioaccumulation in Fish: Aqueous and Dietary Exposure. OECD Guidelines for the Testing of Chemicals. OECD Publishing, Paris. doi:10.1787/9789264185296-en
OECD (2012c). Validation Report (Phase 2) for the Zebrafish Embryo Toxicity Test. OECD Series of Testing and Assessment, No. 179. OECD Publishing, Paris. https://bit.ly/2LFH7Ut
OECD (2013). Test No. 236: Fish Embryo Acute Toxicity (FET) Test. OECD Guidelines for the Testing of Chemicals, Section 2. OECD Publishing, Paris. doi:10.1787/9789264203709-en
OECD (2014). New Guidance Document on an Integrated Approach to Testing and Assessment (IATA) for Skin Corrosion and Irritation. Series of Testing and Assessment, No. 203. OECD Publishing, Paris. https://bit.ly/38cGj1d
OECD (2016). Guidance Document on the Reporting of Defined Approaches to be Used Within Integrated Approaches to Testing and Assessment. OECD Series on Testing and Assessment, No. 255. OECD Publishing, Paris. https://bit.ly/37qsEo2
OECD (2018a). Guidance Document on Integrated Approaches to Testing and Assessment (IATA) for Serious Eye Damage and Eye Irritation. OECD Series on Testing and Assessment, No. 263. OECD Publishing, Paris. doi:10.1787/84b83321-en
OECD (2018b). Guidance Document on Good In Vitro Method Practice (GIVIMP). OECD Series of Testing and Assessment, No. 286. OECD Publishing, Paris. doi:10.1787/9789264304796-21-en
OECD (2019a). Guidance Document on Aqueous-Phase Aquatic Toxicity Testing of Difficult Test Chemicals. OECD Series of Testing and Assessment, No. 23 (second edition). OECD Publishing, Paris. doi:10.1787/0ed2f88e-en
OECD (2019b). Test Guideline No. 203: Fish, Acute Toxicity Testing. OECD Test Guidelines for the Testing of Chemicals, Section 2. OECD Publishing, Paris. doi:10.1787/9789264069961-en
Paparella, M., Colacci, A. and Jacobs, M. N. (2017). Uncertainties of testing methods: What do we (want to) know about carcinogenicity? ALTEX 34, 235-252. doi:10.14573/altex.1608281
Paparella, M., Bennekou, S. H. and Bal-Price, A. (2020). An analysis of the limitations and uncertainties of in vivo developmental neurotoxicity testing and assessment to identify the potential for alternative approaches. Reprod Toxicol 96, 327-336. doi:10.1016/j.reprotox.2020.08.002
Prieto, P., Graepel, R., Gerloff, K. et al. (2019). Investigating cell type specific mechanisms contributing to acute oral toxicity. ALTEX 36, 39-64. doi:10.14573/altex.1805181
Rawlings, J. M., Belanger, S. E., Connors, K. A. et al. (2019). Fish embryo tests and acute fish toxicity tests are interchangeable in the application of the threshold approach. Environ Toxicol Chem 38, 671-681. doi:10.1002/etc.4351
Rufli, H. (2012). Introduction of moribund category to OECD fish acute test and its effect on suffering and LC50 values. Environ Toxicol Chem 31, 1107-1112. doi:10.1002/etc.1779
Russell, W. M. S. and Burch, R. L. (1959). The Principles of Humane Experimental Technique. https://caat.jhsph.edu/principles/the-principles-of-humane-experimental-technique
Schlenk, D., Celander, M., Gallagher, E. et al. (2008). Biotransformation in fishes. In R. T. Di Giulio and D. E. Hinton (eds), The Toxicology of Fishes. Boca Raton, USA: CRC Press. doi:10.1201/9780203647295
Scholz, S., Sela, E., Blaha, L. et al. (2013). A European perspective on alternatives to animal testing for environmental hazard identification and risk assessment. Regul Toxicol Pharmacol 67, 506-530. doi:10.1016/j.yrtph.2013.10.003
Scholz, S., Klüver, N. and Kühne, R. (2016). Analysis of the relevance and adequateness of using Fish Embryo Acute Toxicity (FET) Test Guidance (OECD 236) to fulfil the information requirements and addressing concerns under REACH. Report ECHA-UFZ contract ECHA/2014/341, 1-105.
Stengel, D., Wahby, S. and Braunbeck, T. (2018). In search of a comprehensible set of endpoints for the routine monitoring of neurotoxicity in vertebrates: Sensory perception and nerve transmission in zebrafish (Danio rerio) embryos. Environ Sci Pollut Res Int 25, 4066-4084. doi:10.1007/s11356-017-0399-y
Tanneberger, K., Knobel, M., Busser, F. J. et al. (2013). Predicting fish acute toxicity using a fish gill cell line-based toxicity assay. Environ Sci Technol 47, 1110-1119. doi:10.1021/es303505z
Thomas, P. C., Bicherel, P. and Bauer, F. J. (2019). How in silico and QSAR approaches can increase confidence in environmental hazard and risk assessment. Integr Environ Assess Manag 15, 40-50. doi:10.1002/ieam.4108
US EPA (2001). Final Report: Interlaboratory Variability Study of EPA Short-term Chronic and Acute Whole Effluent Toxicity Test Methods, Vol. 1. EPA 821-B-01-004. Office of Water, U.S. Environmental Protection Agency, Washington, D.C. https://bit.ly/3nraCaU
US EPA (2016). OCSPP 850.1075: Freshwater and Saltwater Fish Acute Toxicity Test. Ecological Effects Test Guidelines. https://www.epa.gov/test-guidelines-pesticides-and-toxic-substances/series-850-ecological-effects-test-guidelines
Vinken, M. and Blaauboer, B. J. (2017). In vitro testing of basal cytotoxicity: Establishment of an adverse outcome pathway from chemical insult to cell death. Toxicol In Vitro 39, 104-110. doi:10.1016/j.tiv.2016.12.004
Volz, D. C., Belanger, S., Embry, M. et al. (2011). Adverse outcome pathways during early fish development: A conceptual framework for identification of chemical screening and prioritization strategies. Toxicol Sci 123, 349-358. doi:10.1093/toxsci/kfr185
WHO – World Health Organization and IPCS – International Programme on Chemical Safety (2018). Guidance document on evaluating and expressing uncertainty in hazard characterization. 2nd edition. World Health Organization. https://apps.who.int/iris/handle/10665/259858
Zhao, Q., De Laender, F. and Van Den Brink, P. J. (2020). Community composition modifies direct and indirect effects of pesticides in freshwater food webs. Sci Total Environ 739, 139531. doi:10.1016/j.scitotenv.2020.139531
Zindler, F., Beedgen, F., Brandt, D. et al. (2019). Analysis of tail coiling activity of zebrafish (Danio rerio) embryos allows for the differentiation of neurotoxicants with different modes of action. Ecotoxicol Environ Saf 186, 109754. doi:10.1016/j.ecoenv.2019.109754