In vitro assessment of tick-borne encephalitis vaccine: Suitable human cell platforms and potential biomarkers
Main Article Content
Abstract
Tick-borne encephalitis (TBE) virus causes a severe disease that can lead to permanent neurological complications. The whole inactivated TBE vaccine is highly effective, as proven by high seroconversion rates and near eradication of the disease in countries where vaccination programs have been implemented. TBE vaccine potency testing currently requires the use of in vivo methods that present issues of reproducibility as well as animal discomfort. As an alternative, public and private entities are currently exploring a batch-to-batch consistency approach that would demonstrate conformity of a newly produced vaccine batch with a batch of proven in vivo efficacy with respect to a range of measurable in vitro quality parameters.
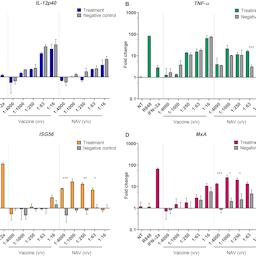
To identify a suitable cellular platform to be used in a panel of in vitro batch-to-batch assessments for the TBE vaccine, we exposed human cell-based systems, both of primary origin and cell line-derived, to vaccine formulations of high and low quality. Following stimulation, cell responses were evaluated by assessing the expression of selected genes by RT-qPCR. Our findings show that the expression of interferon-stimulated genes differed after treatment with non-adjuvanted vaccine batches of different quality in peripheral blood mononuclear cells (PBMCs) and in monocyte-derived dendritic cells, but not in monocyte-free PBMC suspensions nor in cell line-derived immune cells.
These results indicate suitable platforms and potential biomarkers for a cell-based assay that, together with other immunochemical analyses, could serve for batch-to-batch assessment of the TBE vaccine, reducing, and eventually replacing, in vivo methods for potency testing.
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.
Articles are distributed under the terms of the Creative Commons Attribution 4.0 International license (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution and reproduction in any medium, provided the original work is appropriately cited (CC-BY). Copyright on any article in ALTEX is retained by the author(s).
Akkermans, A., Chapsal, J. M., Coccia, E. M. et al. (2020). Animal testing for vaccines. Implementing replacement, reduction and refinement: Challenges and priorities. Biologicals 68, 92-107. doi:10.1016/j.biologicals.2020.07.010
Amicizia, D., Domnich, A., Panatto, D. et al. (2013). Epidemiology of tick-borne encephalitis (TBE) in Europe and its prevention by available vaccines. Human Vaccin Immunother 9, 1163-1171. doi:10.4161/hv.23802
Anderson, J., Toh, Z. Q., Reitsma, A. et al. (2019). Effect of peripheral blood mononuclear cell cryopreservation on innate and adaptive immune responses. J Immunol Methods 465, 61-66. doi:10.1016/J.JIM.2018.11.006
Banchereau, R., Baldwin, N., Cepika, A.-M. et al. (2014). Transcriptional specialization of human dendritic cell subsets in response to microbial vaccines. Nat Commun 5, 5283. doi:10.1038/ncomms6283
Beran, J., Lattanzi, M., Xie, F. et al. (2018). Second five-year follow-up after a booster vaccination against tick-borne encephalitis following different primary vaccination schedules demonstrates at least 10 years antibody persistence. Vaccine 37, 4623-4629. doi:10.1016/J.VACCINE.2017.12.081
Berges, C., Naujokat, C., Tinapp, S. et al. (2005). A cell line model for the differentiation of human dendritic cells. Biochem Biophys Res Commun 333, 896-907. doi:10.1016/j.bbrc.2005.05.171
Bogovic, P. and Strle, F. (2015). Tick-borne encephalitis: A review of epidemiology, clinical characteristics, and management. World J Clin Cases 3, 430-441. doi:10.12998/wjcc.v3.i5.430
Bosshart, H. and Heinzelmann, M. (2016). THP-1 cells as a model for human monocytes. Ann Transl Med 4, 438. doi:10.21037/atm.2016.08.53
Chanput, W., Mes, J. J. and Wichers, H. J. (2014). THP-1 cell line: An in vitro cell model for immune modulation approach. Int Immunopharmacol 23, 37-45. doi:10.1016/j.intimp.2014.08.002
Coombes, L., Tierney, R., Rigsby, P. et al. (2012). In vitro antigen ELISA for quality control of tetanus vaccines. Biologicals 40, 466-472. doi:10.1016/j.biologicals.2012.07.011
Daigneault, M., Preston, J. A., Marriott, H. M. et al. (2010). The identification of markers of macrophage differentiation in PMA-stimulated THP-1 cells and monocyte-derived macrophages. PLoS One 5, e8668. doi:10.1371/journal.pone.0008668
De Mattia, F., Chapsal, J.-M., Descamps, J. et al. (2011). The consistency approach for quality control of vaccines – A strategy to improve quality control and implement 3Rs. Biologicals 39, 59-65. doi:10.1016/j.biologicals.2010.12.001
Dörrbecker, B., Dobler, G., Spiegel, M. et al. (2010). Tick-borne encephalitis virus and the immune response of the mammalian host. Travel Med Infect Dis 8, 213-222. doi:10.1016/j.tmaid.2010.05.010
Drake, D. R., Singh, I., Nguyen, M. N. et al. (2012). In Vitro biomimetic model of the human immune system for predictive vaccine assessments. Disrupt Sci Technol 1, 28-40. doi:10.1089/dst.2012.0006
Estrella, J. L., Kan-Sutton, C., Gong, X. et al. (2011). A novel in vitro human macrophage model to study the persistence of mycobacterium tuberculosis using vitamin D3 and retinoic acid activated THP-1 macrophages. Front Microbiol 2, 67. doi:10.3389/fmicb.2011.00067
European Pharmacopoeia (2008). Tick-borne encephalitis vaccine (inactivated) – Monograph. In European Pharmacopoeia (Ph. Eur.), 8th edition (908-910).
Germann, A., Schulz, J. C., Kemp-Kamke, B. et al. (2011). Standardized serum-free cryomedia maintain peripheral blood mononuclear cell viability, recovery, and antigen-specific T-cell response compared to fetal calf serum-based medium. Biopreserv Biobank 9, 229-236. doi:10.1089/bio.2010.0033
Haglund, M. and Günther, G. (2003). Tick-borne encephalitis – Pathogenesis, clinical course and long-term follow-up. Vaccine 21, Suppl 1, S11-S18. doi:10.1016/S0264-410X(02)00811-3
Hayman, Y. A., Sadofsky, L. R., Williamson, J. D. et al. (2017). The effects of exogenous lipid on THP-1 cells: An in vitro model of airway aspiration? ERJ Open Res 3, 00026-2016. doi:10.1183/23120541.00026-2016
Heinz, F. X., Holzmann, H., Essl, A. et al. (2007). Field effectiveness of vaccination against tick-borne encephalitis. Vaccine 25, 7559-7567. doi:10.1016/j.vaccine.2007.08.024
Hendriksen, C. F. M. (2009). Replacement reduction and refinement alternatives to animal use in vaccine potency measurement. Expert Rev Vaccines 8, 313-322. doi:10.1586/14760584.8.3.313
Herrera-Rodriguez, J., Signorazzi, A., Holtrop, M. et al. (2019). Inactivated or damaged? Comparing the effect of inactivation methods on influenza virions to optimize vaccine production. Vaccine 37, 1630-1637. doi:10.1016/j.vaccine.2019.01.086
Heydenreich, B., Bellinghausen, I., Lund, L. et al. (2014). Adjuvant effects of aluminium hydroxide-adsorbed allergens and allergoids – Differences in vivo and in vitro. Clin Exp Immunol 176, 310-319. doi:10.1111/cei.12294
Higashi, T., Wakui, M., Nakano, K. et al. (2008). Evaluation of adjuvant activities using human antigen presenting cells in vitro. Allergol Int 57, 219-222. doi:10.2332/allergolint.O-07-523
Hoefnagel, M. H. N., Vermeulen, J. P., Scheper, R. J. et al. (2011). Response of MUTZ-3 dendritic cells to the different components of the Haemophilus influenzae type B conjugate vaccine: Towards an in vitro assay for vaccine immunogenicity. Vaccine 29, 5114-5121. doi:10.1016/j.vaccine.2011.05.050
Holzmann, H., Karganova, G., Barrett, A. et al. (2011). Background document on vaccines and vaccination against tick-borne encephalitis (TBE). http://www.who.int/immunization/sage/6_TBE_backgr_18_Mar_net_apr_2011.pdf
Hoonakker, M. E., Verhagen, L. M., Hendriksen, C. F. et al. (2015). In vitro innate immune cell based models to assess whole cell Bordetella pertussis vaccine quality: A proof of principle. Biologicals 43, 100-109. doi:10.1016/j.biologicals.2014.12.002
Janetzki, S., Price, L., Britten, C. M. et al. (2010). Performance of serum-supplemented and serum-free media in IFNy Elispot assays for human T cells. Cancer Immunol Immunother 59, 609-618. doi:10.1007/s00262-009-0788-2
Jeon, M. K., Lim, J. B. and Lee, G. M. (2010). Development of a serum-free medium for in vitro expansion of human cytotoxic T lymphocytes using a statistical design. BMC Biotechnol 10, 70. doi:10.1186/1472-6750-10-70
Ko, E.-J., Lee, Y.-T., Kim, K.-H. et al. (2017). Roles of aluminum hydroxide and monophosphoryl lipid A adjuvants in overcoming CD4+ T cell deficiency to induce isotype-switched IgG antibody responses and protection by T-dependent influenza vaccine. J Immunol 198, 279-291. doi:10.4049/jimmunol.1600173
Kooijman, S., Brummelman, J., van Els, C. A. C. M. et al. (2018). Vaccine antigens modulate the innate response of monocytes to Al(OH)3. PLoS One 13, e0197885. doi:10.1371/journal.pone.0197885
Kubinski, M., Beicht, J., Gerlach, T. et al. (2020). Tick-borne encephalitis virus: A quest for better vaccines against a virus on the rise. Vaccines 8, 451. doi:10.3390/vaccines8030451
Lang, C., Kolaj-Robin, O., Cirefice, G. et al. (2018). Replacement, reduction, refinement – Animal welfare progress in European Pharmacopoeia monographs: Activities of the European Pharmacopoeia Commission from 2007 to 2017. Pharmeur Bio Sci Notes 2018, 12-36. https://bit.ly/2TPY6HT
Leenaars, P. P. A., Kersten, G. F., de Bruijn, M. L. et al. (2001). An in vitro approach in quality control of toxoid vaccines. Vaccine 19, 2729-2733. doi:10.1016/S0264-410X(00)00510-7
Lehrer, A. T. and Holbrook, M. R. (2011). Tick-borne encephalitis vaccines. J Bioterror Biodef 2011, Suppl 1, 3. doi:10.4172/2157-2526.S1-003
Leist, M. and Hartung, T. (2013). Inflammatory findings on species extrapolations: Humans are definitely no 70-kg mice. Arch Toxicol 87, 563-567. doi:10.1007/s00204-013-1038-0
Lindqvist, R., Upadhyay, A. and Överby, A. K. (2018). Tick-borne flaviviruses and the type I interferon response. Viruses 10, 340. doi:10.3390/v10070340
Martikainen, M.-V. and Roponen, M. (2020). Cryopreservation affected the levels of immune responses of PBMCs and antigen-presenting cells. Toxicol In Vitro 67, 104918. doi:10.1016/j.tiv.2020.104918
Ming, M., Bernardo, L., Williams, K. et al. (2019). An in vitro functional assay to measure the biological activity of TB vaccine candidate H4-IC31. Vaccine 37, 2960-2966. doi:10.1016/j.vaccine.2019.04.035
Minor, P. D. (2015). Assaying the potency of influenza vaccines. Vaccines 3, 90-104. doi:10.3390/vaccines3010090
Mold, M., Shardlow, E. and Exley, C. (2016). Insight into the cellular fate and toxicity of aluminium adjuvants used in clinically approved human vaccinations. Sci Rep 6, 31578. doi:10.1038/SREP31578
Nah, K., Bede-Fazekas, Á., Trájer, A. J. et al. (2020). The potential impact of climate change on the transmission risk of tick-borne encephalitis in Hungary. BMC Infect Dis 20, 34. doi:10.1186/s12879-019-4734-4
Nelissen, I., Selderslaghs, I., Van Den Heuvel, R. et al. (2009). MUTZ-3-derived dendritic cells as an in vitro alternative model to CD34+ progenitor-derived dendritic cells for testing of chemical sensitizers. Toxicol In Vitro 23, 1477-1481. doi:10.1016/j.tiv.2009.08.022
Orlinger, K. K., Hofmeister, Y., Fritz, R. et al. (2011). A tick-borne encephalitis virus vaccine based on the European prototype strain induces broadly reactive cross-neutralizing antibodies in humans. J Infect Dis 203, 1556-1564. doi:10.1093/infdis/jir122
Overby, A. K., Popov, V. L., Niedrig, M. et al. (2010). Tick-borne encephalitis virus delays interferon induction and hides its double-stranded RNA in intracellular membrane vesicles. J Virol 84, 8470-8483. doi:10.1128/jvi.00176-10
Petrovsky, N. (2015). Comparative safety of vaccine adjuvants: A summary of current evidence and future needs. Drug Saf 38, 1059-1074. doi:10.1007/s40264-015-0350-4
Radke, L., López Hemmerling, D. A., Lubitz, A. et al. (2012). Induced cytokine response of human PMBC-cultures: Correlation of gene expression and secretion profiling and the effect of cryopreservation. Cell Immunol 272, 144-153. doi:10.1016/j.cellimm.2011.10.018
Růžek, D., Avšič Županc, T., Borde, J. et al. (2019). Tick-borne encephalitis in Europe and Russia: Review of pathogenesis, clinical features, therapy, and vaccines. Antiviral Res 164, 23-51. doi:10.1016/j.antiviral.2019.01.014
Ryu, W.-S. (2017). Chapter 4 – Diagnosis and Methods. In W.-S. Ryu (ed.), Molecular Virology of Human Pathogenic Viruses (47-62). Academic Press. doi:10.1016/B978-0-12-800838-6.00004-7
Safar, R., Doumandji, Z., Saidou, T. et al. (2019). Cytotoxicity and global transcriptional responses induced by zinc oxide nanoparticles NM 110 in PMA-differentiated THP-1 cells. Toxicol Lett 308, 65-73. doi:10.1016/j.toxlet.2018.11.003
Sasaki, E., Momose, H., Hiradate, Y. et al. (2018). In vitro marker gene expression analyses in human peripheral blood mononuclear cells: A tool to assess safety of influenza vaccines in humans. J Immunotoxicol 15, 53-62. doi:10.1080/1547691X.2018.1447052
Schmittgen, T. D. and Livak, K. J. (2008). Analyzing real-time PCR data by the comparative CT method. Nat Protoc 3, 1101-1108. doi:10.1038/nprot.2008.73
Schutte, K., Szczepanska, A., Halder, M. et al. (2017). Modern science for better quality control of medicinal products “Towards global harmonization of 3Rs in biologicals”: The report of an EPAA workshop. Biologicals 48, 55-65. doi:10.1016/j.biologicals.2017.05.006
Solati, S., Aarden, L., Zeerleder, S. et al. (2015). An improved monocyte activation test using cryopreserved pooled human mononuclear cells. Innate Immun 21, 677-684. doi:10.1177/1753425915583365
Starr, T., Bauler, T. J., Malik-Kale, P. et al. (2018). The phorbol 12-myristate-13-acetate differentiation protocol is critical to the interaction of THP-1 macrophages with Salmonella Typhimurium. PLoS One 13, e0193601. doi:10.1371/journal.pone.0193601
Stoel, M., Pool, J., de Vries-Idema, J. et al. (2015). Innate responses induced by whole inactivated virus or subunit influenza vaccines in cultured dendritic cells correlate with immune responses in vivo. PloS One 10, e0125228. doi:10.1371/journal.pone.0125228
Süss, J. (2011). Tick-borne encephalitis 2010: Epidemiology, risk areas, and virus strains in Europe and Asia – An overview. Ticks Tick Borne Dis 2, 2-15. doi:10.1016/j.ttbdis.2010.10.007
Tapia-Calle, G., Stoel, M., de Vries-Idema, J. et al. (2017). Distinctive responses in an in vitro human dendritic cell-based system upon stimulation with different influenza vaccine formulations. Vaccines 5, 21. doi:10.3390/vaccines5030021
Trück, J., Mitchell, R., Thompson, A. J. et al. (2014). Effect of cryopreservation of peripheral blood mononuclear cells (PBMCs) on the variability of an antigen-specific memory B cell ELISpot. Hum Vaccin Immunother 10, 2490-2496. doi:10.4161/hv.29318
Van Voorhis, W., Hair, L., Steinman, R. et al. (1982). Human dendritic cells. Enrichment and characterization from peripheral blood. J Exp Med 155, 1172-1187. doi:10.1084/jem.155.4.1172
Vandebriel, R. J. and Hoefnagel, M. H. N. (2012). Dendritic cell-based in vitro assays for vaccine immunogenicity. Hum Vaccin Immunother 8, 1323-1325. doi:10.4161/hv.21350
Verthelyi, D., Casey, W., Arciniega, J. et al. (2011). Non-animal replacement methods for human vaccine potency testing: State of the science and future directions. Procedia Vaccinol 5, 16-32. doi:10.1016/j.provac.2011.10.002
Wei, J., Kang, X., Li, Y. et al. (2013). Pathogenicity of tick-borne encephalitis virus to monocytes [Article in Chinese]. Wei Sheng Wu Xue Bao 53, 1221-1225.
Wieczorek, L., Brown, B. K., DelSarto Macedo, C. et al. (2013). Mitigation of variation observed in a peripheral blood mononuclear cell (PBMC) based HIV-1 neutralization assay by donor cell pooling. Virology 447, 240-248. doi:10.1016/J.VIROL.2013.09.014
WHO – World Health Organization Expert Committee on Biological Standardization (2014). Guidelines on the nonclinical evaluation of vaccine adjuvants and adjuvanted vaccines. In WHO Technical Report Series 987. https://bit.ly/3vK1ZLK
Yang, Q., You, J., Zhou, Y. et al. (2020). Tick-borne encephalitis virus NS4A ubiquitination antagonizes type I interferon-stimulated STAT1/2 signalling pathway. Emerg Microbes Infect 9, 714-726. doi:10.1080/22221751.2020.1745094
Zhang, J., Shao, J., Wu, X. et al. (2015). Type I interferon related genes are common genes on the early stage after vaccination by meta-analysis of microarray data. Hum Vaccin Immunother 11, 739-745. doi:10.1080/21645515.2015.1008884
Zhang, X., Zheng, Z., Liu, X. et al. (2016). Tick-borne encephalitis virus induces chemokine RANTES expression via activation of IRF-3 pathway. J Neuroinflammation 13, 209. doi:10.1186/s12974-016-0665-9