A microfluidic thyroid-liver platform to assess chemical safety in humans
Main Article Content
Abstract
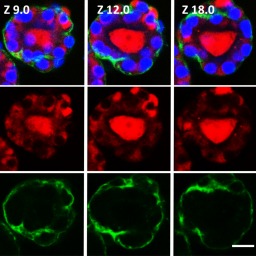
Thyroid hormones (THs) are crucial regulators of human metabolism and early development. During the safety assessment of plant protection products, the human relevance of chemically induced TH perturbations observed in test animals remains uncertain. European regulatory authorities request follow-up in vitro studies to elucidate human-relevant interferences on thyroid gland function or TH catabolism through hepatic enzyme induction. However, human in vitro assays based on single molecular initiating events poorly reflect the complex TH biology and related liver-thyroid axis. To address this complexity, we present human three-dimensional thyroid and liver organoids with key functions of TH metabolism. The thyroid model resembles in vivo-like follicular architecture and a TSH-dependent triiodothyronine synthesis over 21 days, which is inhibited by methimazole. The HepaRG-based liver model, secreting the critical TH-binding proteins albumin and thyroxine-binding globulin, emulates an active TH catabolism via the formation of glucuronidated and sulfated thyroxine (gT4/sT4). Activation of the nuclear receptors PXR and AHR was demonstrated via the induction of specific CYP isoenzymes by rifampicin, pregnenolone-16α-carbonitrile, and β-naphthoflavone. However, this nuclear receptor activation, assumed to regulate UDP-glucuronosyltransferases and sulfotransferases, appeared to have no effect on gT4 and sT4 formation in this human-derived hepatic cell line model. Finally, established single-tissue models were successfully co-cultured in a perfused two-organ chip for 21 days. In conclusion, this model presents a first step towards a complex multimodular human platform that will help to identify both direct and indirect thyroid disruptors that are relevant from a human safety perspective.
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.
Articles are distributed under the terms of the Creative Commons Attribution 4.0 International license (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution and reproduction in any medium, provided the original work is appropriately cited (CC-BY). Copyright on any article in ALTEX is retained by the author(s).
Abel, E. D., Ahima, R. S., Boers, M.-E. et al. (2001). Critical role for thyroid hormone receptor β2 in the regulation of paraventricular thyrotropin-releasing hormone neurons. J Clin Invest 107, 1017-1023. doi:10.1172/JCI10858
Abu-Absi, S. F., Hansen, L. K. and Hu, W.-S. (2004). Three-dimensional co-culture of hepatocytes and stellate cells. Cytotechnology 45, 125-140. doi:10.1007/s10616-004-7996-6
Al-Salman, F. and Plant, N. (2012). Non-coplanar polychlorinated biphenyls (PCBs) are direct agonists for the human pregnane-X receptor and constitutive androstane receptor, and activate target gene expression in a tissue-specific manner. Toxicol Appl Pharmacol 263, 7-13. doi:10.1016/j.taap.2012.05.016
Aninat, C., Piton, A., Glaise, D. et al. (2006). Express ion of cytochromes P450, conjugating enzymes and nuclear receptors in human hepatoma HepaRG cells. Drug Metab Dispos 34, 75-83. doi:10.1124/dmd.105.006759
Bartalena, L. and Robbins, J. (1992). Variations in thyroid hormone transport proteins and their clinical implications. Thyroid 2, 237-245. doi:10.1089/thy.1992.2.237
Bartsch, R., Brinkmann, B., Jahnke, G. et al. (2018). Human relevance of follicular thyroid tumors in rodents caused by non-genotoxic substances. Regul Toxicol Pharmacol 98, 199-208. doi:10.1016/j.yrtph.2018.07.025
Bassett, J. H. D. and Williams, G. R. (2003). The molecular actions of thyroid hormone in bone. Trends Endocrinol Metab 14, 356-364. doi:10.1016/S1043-2760(03)00144-9
Bauer, S., Wennberg Huldt, C., Kanebratt, K. P. et al. (2017). Functional coupling of human pancreatic islets and liver spheroids on-a-chip: Towards a novel human ex vivo type 2 diabetes model. Sci Rep 7, 14620. doi:10.1038/s41598-017-14815-w
Beilmann, M., Boonen, H., Czich, A. et al. (2019). Optimizing drug discovery by investigative toxicology: Current and future trends. ALTEX 36, 289-313. doi:10.14573/altex.1808181
Bell, C. C., Hendriks, D. F. G., Moro, S. M. L. et al. (2016). Characterization of primary human hepatocyte spheroids as a model system for drug-induced liver injury, liver function and disease. Sci Rep 6, 25187. doi:10.1038/srep25187
Bernal, J. (2007). Thyroid hormone receptors in brain development and function. Nat Clin Prac Endocrinol Metab 3, 249-259. doi:10.1038/ncpendmet0424
Bürgi-Saville, M. E., Gerber, H., Peter, H. J. et al. (1997). Expression patterns of extracellular matrix components in native and cultured normal human thyroid tissue and in human toxic adenoma tissue. Thyroid 7, 347-356. doi:10.1089/thy.1997.7.347
Chambard, M., Gabrion, J. and Mauchamp, J. (1981). Influence of collagen gel on the orientation of epithelial cell polarity: Follicle formation from isolated thyroid cells and from preformed monolayers. J Cell Biol 91, 157-166. doi:10.1083/jcb.91.1.157
Chambard, M., Verrier, B., Gabrion, J. et al. (1984). Polarity reversal of inside‐out thyroid follicles cultured within collagen gel: Reexpression of specific functions. Biol Cell 51, 315-325. doi:10.1111/j.1768-322X.1984.tb00310.x
Coecke, S., Balls, M., Bowe, G. et al. (2005). Guidance on good cell culture practice – A report of the second ECVAM task force on good cell culture practice. Altern Lab Anim 33, 261-287. doi:10.1177/026119290503300313
Cox, C. R., Lynch, S., Goldring, C. et al. (2020). Current perspective: 3D spheroid models utilizing human-based cells for investigating metabolism-dependent drug-induced liver injury. Front Med Technol 2, 611913. doi:10.3389/fmedt.2020.611913
Crivellente, F., Hart, A., Hernandez-Jerez, A. F. et al. (2019). Establishment of cumulative assessment groups of pesticides for their effects on the thyroid. EFSA J 17, e05801. doi:10.2903/j.efsa.2019.5801
Curran, P. G. and DeGroot, L. J. (1991). The effect of hepatic enzyme-inducing drugs on thyroid hormones and the thyroid gland. Endocr Rev 12, 135-150. doi:10.1210/edrv-12-2-135
Darnell, M., Ulvestad, M., Ellis, E. et al. (2012). In vitro evaluation of major in vivo drug metabolic pathways using primary human hepatocytes and HepaRG cells in suspension and a dynamic three-dimensional bioreactor system. J Pharmacol Exp Ther 343, 134-144. doi:10.1124/jpet.112.195834
Deisenroth, C., Soldatow, V. Y., Ford, J. et al. (2020). Development of an in vitro human thyroid microtissue model for chemical screening. Toxicol Sci 174, 63-78. doi:10.1093/toxsci/kfz238
Desai, P. K., Tseng, H. and Souza, G. R. (2017). Assembly of hepatocyte spheroids using magnetic 3D cell culture for CYP450 inhibition/induction. Int J Mol Sci 18, 1085. doi:10.3390/ijms18051085
DeVito, M., Biegel, L., Brouwer, A. et al. (1999). Screening methods for thyroid hormone disrupters. Environ Health Perspect 107, 407-415. doi:10.1289/ehp.99107407
Domingues, R., Font, P., Sobrinho, L. et al. (2009). A novel variant in Serpina7 gene in a family with thyroxine-binding globulin deficiency. Endocrine 36, 83-86. doi:10.1007/s12020-009-9202-2
Dumont, J. E. (1971). The action of thyrotropin on thyroid metabolism. Vitam Horm 29, 287-412. doi:10.1016/S0083-6729(08)60051-5
Elaut, G., Henkens, T., Papeleu, P. et al. (2006). Molecular mechanisms underlying the dedifferentiation process of isolated hepatocytes and their cultures. Curr Drug Metab 7, 629-660. doi:10.2174/138920006778017759
EC – European Commission, Directorate-General for Environment (2017). Supporting the organisation of a workshop on thyroid disruption – Final report. Publications Office. doi:10.2779/921523
ECHA and EFSA, Joint Research Centre et al. (2018). Guidance for the identification of endocrine disruptors in the context of Regulations (EU) No 528/2012 and (EC) No 1107/2009. EFSA J 16, 1-135. doi:10.2903/j.efsa.2018.5311
Felmlee, D. J., Grün, D. and Baumert, T. F. (2018). Zooming in on liver zonation. Hepatology 67, 784-787. doi:10.1002/hep.29554
Findlay, K. A. B., Kaptein, E., Visser, T. J. et al. (2000). Characterization of the uridine diphosphate-glucuronosyltransferase-catalyzing thyroid hormone glucuronidation in man. J Clin Endocrinol Metabol 85, 2879-2883. doi:10.1210/jc.85.8.2879
Foster, J. R., Tinwell, H. and Melching-Kollmuss, S. (2021). A review of species differences in the control of, and response to, chemical-induced thyroid hormone perturbations leading to thyroid cancer. Arch Toxicol 95, 807-836. doi:10.1007/s00204-020-02961-6
Friedman, K. P., Watt, E. D., Hornung, M. W. et al. (2016). Tiered high-throughput screening approach to identify thyroperoxidase inhibitors within the toxcast phase I and II chemical libraries. Toxicol Sci 151, 160-180. doi:10.1093/toxsci/kfw034
Friesema, E. C. H., Ganguly, S., Abdalla, A. et al. (2003). Identification of monocarboxylate transporter 8 as a specific thyroid hormone transporter. J Biol Chem 278, 40128-40135. doi:10.1074/jbc.M300909200
Friesema, E. C. H., Jansen, J., Milici, C. et al. (2005). Thyroid hormone transporters. Vitam Horm 70, 137-167. doi:10.1016/S0083-6729(05)70005-4
Fukuchi, M., Shimabukuro, M., Shimajiri, Y. et al. (2002). Evidence for a deficient pancreatic β-cell response in a rat model of hyperthyroidism. Life Sci 71, 1059-1070. doi:10.1016/S0024-3205(02)01791-5
Gamage, N., Barnett, A., Hempel, N. et al. (2006). Human sulfotransferases and their role in chemical metabolism. Toxicol Sci 90, 5-22. doi:10.1093/toxsci/kfj061
Garbi, C., Tacchetti, C. and Wollman, S. H. (1986). Change of inverted thyroid follicle into a spheroid after embedding in a collagen gel. Exp Cell Res 163, 63-77. doi:10.1016/0014-4827(86)90558-6
Gardas, A. (1991). Laboratory tests: Level of T3, T4, TSH and antithyroid gland autoantibodies in the Polish population [Article in Polish]. Endokrynol Pol 42, 353-358.
Gaskell, H. J. (2016). Development of a Spheroid Model to Investigate Drug-Induced Liver Injury (Thesis). University of Liverpool, UK. https://livrepository.liverpool.ac.uk/3004330/1/200648599_Aug2016.pdf
Hammond, G. L., Hill, L. A. and Round, P. W. (2019). Roles of plasma binding proteins in modulation of hormone action and metabolism. In I. Huhtaniemi and L. Martini (eds), Encyclopedia of Endocrine Diseases (51-60). 2nd edition. doi:10.1016/B978-0-12-801238-3.64186-7
Goodman, H. M. (2009). Chapter 3 – Thyroid gland. In H. M. Goodman (ed.), Goodman’s Basic Medical Endocrinology (43-59). 4th edition. Elsevier. doi:10.1016/B978-0-12-373975-9.00003-3
Gunness, P., Mueller, D., Shevchenko, V. et al. (2013). 3D organotypic cultures of human HepaRG cells: A tool for in vitro toxicity studies. Toxicol Sci 133, 67-78. doi:10.1093/toxsci/kft021
Hallgren, S. and Darnerud, P. O. (2002). Polybrominated diphenyl ethers (PBDEs), polychlorinated biphenyls (PCBs) and chlorinated paraffins (CPs) in rats – Testing interactions and mechanisms for thyroid hormone effects. Toxicology 177, 227-243. doi:10.1016/s0300-483x(02)00222-6
Hendriks, D. F. G., Puigvert, L. F., Messner, S. et al. (2016). Hepatic 3D spheroid models for the detection and study of compounds with cholestatic liability. Sci Rep 6, 35434. doi:10.1038/srep35434
Hoekstra, R., Nibourg, G. A. A., Van Der Hoeven, T. V. et al. (2013). Phase 1 and phase 2 drug metabolism and bile acid production of HepaRG cells in a bioartificial liver in absence of dimethyl sulfoxide. Drug Metab Dispos 41, 562-567. doi:10.1124/dmd.112.049098
Hohtari, H., Pakarinen, A. and Kauppila, A. (1987). Serum concentrations of thyrotropin, thyroxine, triiodothyronine and thyroxine binding globulin in female endurance runners and joggers. Acta Endocrinol 114, 41-46. doi:10.1530/acta.0.1140041
Hood, A., Allen, M. L., Liu, Y. P. et al. (2003). Induction of T4 UDP-GT activity, serum thyroid stimulating hormone, and thyroid follicular cell proliferation in mice treated with microsomal enzyme inducers. Toxicol Appl Pharmacol 188, 6-13. doi:10.1016/S0041-008X(02)00071-6
Huaman, C., Caron, S., Briand, O. et al. (2012). The human hepatocyte cell lines IHH and HepaRG: Models to study glucose, lipid and lipoprotein metabolism. Arch Physiol Biochem 118, 102-111. doi:10.3109/13813455.2012.683442
Ito, M., Toyoda, N., Nomura, E. et al. (2011). Type 1 and type 2 iodothyronine deiodinases in the thyroid gland of patients with 3,5,3′-triiodothyronine-predominant Graves’ disease. Eur J Endocrinol 164, 95-100. doi:10.1530/EJE-10-0736
Janssen, S. T. and Janssen, O. E. (2017). Directional thyroid hormone distribution via the blood stream to target sites. Mol Cell Endocrinol 458, 16-21. doi:10.1016/j.mce.2017.02.037
Jomaa, B., De Haan, L. H. J., Peijnenburg, A. A. C. M. et al. (2015). Simple and rapid in vitro assay for detecting human thyroid peroxidase disruption. ALTEX 32, 191-200. doi:10.14573/altex.1412201
Jungsuwadee, P. and Vore, M. E. (2010). 4.26 – Efflux transporters. In C. A. McQueen (ed.), Comprehensive Toxicology (557-601). 2nd edition. Elsevier. doi:10.1016/B978-0-08-046884-6.00426-7
Kammerer, S. and Küpper, J.-H. (2018). Human hepatocyte systems for in vitro toxicology analysis. J Cell Biotechnol 3, 85-93. doi:10.3233/jcb-179012
Kanebratt, K. P., Janefeldt, A., Vilén, L. et al. (2021). Primary human hepatocyte spheroid model as a 3D in vitro platform for metabolism studies. J Pharm Sci 110, 422-431. doi:10.1016/j.xphs.2020.10.043
Karwelat, D., Kuehnlenz, J., Steger-Hartmann, T. et al. (2022). A rodent thyroid-liver chip to capture thyroid toxicity on organ functional level. ALTEX, online ahead of print. doi:10.14573/altex.2108262
Kato, Y., Ikushiro, S. I., Emi, Y. et al. (2008). Hepatic UDP-glucuronosyltransferases responsible for glucuronidation of thyroxine in humans. Drug Metab Dispos 36, 51-55. doi:10.1124/dmd.107.018184
Kester, M. H. A., van Dijk, C. H., Tibboel, D. et al. (1999). Sulfation of thyroid hormone by estrogen sulfotransferase. J Clin Endocrinol Metab 84, 2577-2580. doi:10.1210/jcem.84.7.5975
Kraiem, Z., Sadeh, O. and Yosef, M. (1991). Iodide uptake and organification, tri-iodothyronine secretion, cyclic AMP accumulation and cell proliferation in an optimized system of human thyroid follicles cultured in collagen gel suspended in serum-free medium. J Endocrinol 131, 499-506. doi:10.1677/joe.0.1310499
Kühnl, J., Tao, T. P., Brandmair, K. et al. (2021). Characterization of application scenario-dependent pharmacokinetics and pharmacodynamic properties of permethrin and hyperforin in a dynamic skin and liver multi-organ-chip model. Toxicology 448, 152637. doi:10.1016/j.tox.2020.152637
Kusunoki, T., Nishida, S., Koezuka, M. et al. (2001). Morphological and functional differentiation of human thyroid cells in collagen gel culture. Auris Nasus Larynx 28, 333-338. doi:10.1016/S0385-8146(01)00109-2
Langan, L. M., Dodd, N. J. F., Owen, S. F. et al. (2016). Direct measurements of oxygen gradients in spheroid culture system using electron parametric resonance oximetry. PLoS One 11, e0149492. doi:10.1371/journal.pone.0149492
Lauschke, V. M., Hendriks, D. F. G., Bell, C. C. et al. (2016). Novel 3D culture systems for studies of human liver function and assessments of the hepatotoxicity of drugs and drug candidates. Chem Res Toxicol 29, 1936-1955. doi:10.1021/acs.chemrestox.6b00150
Leemans, M., Couderq, S., Demeneix, B. et al. (2019). Pesticides with potential thyroid hormone-disrupting effects: A review of recent data. Front Endocrinol 10, 743. doi:10.3389/fendo.2019.00743
Leite, S. B., Wilk-Zasadna, I., Zaldivar, J. M. et al. (2012). Three-dimensional HepaRG model as an attractive tool for toxicity testing. Toxicol Sci 130, 106-116. doi:10.1093/toxsci/kfs232
Li, J., Settivari, R. S., Lebaron, M. J. et al. (2019). Functional comparison of HepaRG Cells and primary human hepatocytes in sandwich and spheroid culture as repeated-exposure models for hepatotoxicity. Appl In Vitro Toxicol 5, 187-195. doi:10.1089/aivt.2019.0008
Lübberstedt, M., Müller-Vieira, U., Mayer, M. et al. (2011). HepaRG human hepatic cell line utility as a surrogate for primary human hepatocytes in drug metabolism assessment in vitro. J Pharmacol Toxicol Methods 63, 59-68. doi:10.1016/j.vascn.2010.04.013
Mandon, M., Huet, S., Dubreil, E. et al. (2019). Three-dimensional HepaRG spheroids as a liver model to study human genotoxicity in vitro with the single cell gel electrophoresis assay. Sci Rep 9, 10548. doi:10.1038/s41598-019-47114-7
Marchesini, G. R., Meimaridou, A., Haasnoot, W. et al. (2008) Biosensor discovery of thyroxine transport disrupting chemicals. Toxicol Appl Pharmacol 232, 150-160. doi:10.1016/j.taap.2008.06.014
Marx, U., Akabane, T., Andersson, T. B. et al. (2020). Biology-inspired microphysiological systems to advance patient benefit and animal welfare in drug development. ALTEX 37, 364-394. doi:10.14573/altex.2001241
Massart, C., Hody, B., Condé, D. et al. (1988). Functional properties of human thyroid follicles cultured within collagen gel. Mol Cell Endocrinol 56, 227-234. doi:10.1016/0303-7207(88)90065-2
Mauchamp, J., Mirrione, A., Alquier, C. et al. (1998). Follicle-like structure and polarized monolayer: Role of the extracellular matrix on thyroid cell organization in primary culture. Biol Cell 90, 369-380. doi:10.1016/S0248-4900(98)80086-5
Meek, M. E., Bucher, J. R., Cohen, S. M. et al. (2003). A framework for human relevance analysis of information on carcinogenic modes of action. Crit Rev Toxicol 33, 591-653. doi:10.1080/713608373
Miyawaki, I., Tamura, A., Matsumoto, I. et al. (2012). The effects of clobazam treatment in rats on the expression of genes and proteins encoding glucronosyltransferase 1A/2B (UGT1A/2B) and multidrug resistance‐associated protein-2 (MRP2), and development of thyroid follicular cell hypertrophy. Toxicol Appl Pharmacol 265, 351-359. doi:10.1016/j.taap.2012.09.003
Mondal, S., Raja, K., Schweizer, U. et al. (2016). Chemistry and biology in the biosynthesis and action of thyroid hormones. Angew Chem Int Ed Engl 55, 7606-7630. doi:10.1002/anie.201601116
Murakami, M., Araki, O., Hosoi, Y. et al. (2001). Expression and regulation of type II iodothyronine deiodinase in human thyroid gland. Endocrinology 142, 2961-2967. doi:10.1210/endo.142.7.8280
Murk, A. T. J., Rijntjes, E., Blaauboer, B. J. et al. (2013). Mechanism-based testing strategy using in vitro approaches for identification of thyroid hormone disrupting chemicals. Toxicol In Vitro 27, 1320-1346. doi:10.1016/j.tiv.2013.02.012
Nagasaka, A. and Hidaka, H. (1976). Effect of antithyroid agents 6-propyl-2-thiouracil and l-methyl-2-mercaptoimidazole on human thyroid iodide peroxidase. J Clin Endocrinol Metab 43, 152-158. doi:10.1210/jcem-43-1-152
Nikiforov, Y. E., Biddinger, P. W. and Thompson L. D. R. (2018). Diagnostic Pathology and Molecular Genetics of the Thyroid: A Comprehensive Guide for Practicing Thyroid Pathology. 3rd edition. Lippincott Williams & Wilkins.
Nikrodhanond, A. A., Ortiga-Carvalho, T. M., Shibusawa, N. et al. (2006). Dominant role of thyrotropin-releasing hormone in the hypothalamic- pituitary-thyroid axis. J Biol Chem 281, 5000-5007. doi:10.1074/jbc.M511530200
Nishida, S., Hosokawa, K., Kusunoki, T. et al. (1993). Morphological properties of human thyroid tumor cells in collagen gel culture and metastatic or invasive ability. Histol Histopathol 8, 329-337.
Noyes, P. D., Friedman, K. P., Browne, P. et al. (2019). Evaluating chemicals for thyroid disruption: Opportunities and challenges with in vitro testing and adverse outcome pathway approaches. Environ Health Perspect 127, 95001. doi:10.1289/EHP5297
Ohnhaus, E. and Studer, H. (1983). A link between liver microsomal enzyme activity and thyroid hormone metabolism in man. Br J Clin Pharmacol 15, 71-76. doi:10.1111/j.1365-2125.1983.tb01466.x
Ohtsuki, S., Schaefer, O., Kawakami, H. et al. (2012). Simultaneous absolute protein quantification of transporters, cytochromes P450, and UDP-glucuronosyltransferases as a novel approach for the characterization of individual human liver: Comparison with mRNA levels and activities. Drug Metab Dispos 40, 83-92. doi:10.1124/dmd.111.042259
Okamura, Y., Shigemasa, C. and Tatsuhara, T. (1986). Pharmacokinetics of methimazole in normal subjects and hyperthyroid patients. Endocrinol Jpn 33, 605-615. doi:10.1507/endocrj1954.33.605
Ollis, C. A., Fowles, A., Brown, B. L. et al. (1985). Human thyroid cells in monolayer retain the ability to secrete tri-iodothyronine in response to thyrotrophin. J Endocrinol 104, 285-290. doi:10.1677/joe.0.1040285
Patel, J., Landers, K., Li, H. et al. (2011). Thyroid hormones and fetal neurological development. J Endocrinol 209, 1-8. doi:10.1530/JOE-10-0444
Pilo, A., Ferdeghini, M., Iervasi, G. et al. (1990). Thyroidal and peripheral production of 3,5,3′-triiodothyronine in human by multicompartmental analysis. Am J Physiol 258, E715-126. doi:10.1152/ajpendo.1990.258.4.E715
Ramaiahgari, S. C., Waidyanatha, S., Dixon, D. et al. (2017). Three-dimensional (3D) HepaRG spheroid model with physiologically relevant xenobiotic metabolism competence and hepatocyte functionality for liver toxicity screening. Toxicol Sci 159, 124-136. doi:10.1093/toxsci/kfx122
Richardson, V. M., Ferguson, S. S., Sey, Y. M. et al. (2014). In vitro metabolism of thyroxine by rat and human hepatocytes. Xenobiotica 44, 391-403. doi:10.3109/00498254.2013.847990
Richter, L. H. J., Kaminski, Y. R., Noor, F. et al. (2016). Metabolic fate of desomorphine elucidated using rat urine, pooled human liver preparations, and human hepatocyte cultures as well as its detectability using standard urine screening approaches. Anal Bioanal Chem 408, 6283-6294. doi:10.1007/s00216-016-9740-4
Rouquié, D., Tinwell, H., Blanck, O. et al. (2014). Thyroid tumor formation in the male mouse induced by fluopyram is mediated by activation of hepatic CAR/PXR nuclear receptors. Regul Toxicol Pharmacol 70, 673-680. doi:10.1016/j.yrtph.2014.10.003
Saito, Y., Onishi, N., Takami, H. et al. (2018). Development of a functional thyroid model based on an organoid culture system. Biochem Biophy Res Commun 497, 783-789. doi:10.1016/j.bbrc.2018.02.154
Sasaki, M., Sawada, N., Minase, T. et al. (1991). Collagen-gel-embedded three-dimensional culture of human thyroid epithelial cells: Comparison between the floating sandwich method and the dispersed embedding method. Cell Struct Funct 16, 209-215. doi:10.1247/csf.16.209
Schimek, K., Frentzel, S., Luettich, K. et al. (2020). Human multi-organ chip co-culture of bronchial lung culture and liver spheroids for substance exposure studies. Sci Rep 10, 7865. doi:10.1038/s41598-020-64219-6
Sinha, R. A., Singh, B. K., Yen, P. M. et al. (2018). Direct effects of thyroid hormones on hepatic lipid metabolism. Nat Rev Endocrinol 14, 259-269. doi:10.1038/nrendo.2018.10
Spinel-Gomez, C., Colin, I., van den Hove, M. F. et al. (1990). Correlated morphological and functional study of isolated rat thyroid follicles in suspension culture. Mol Cell Endocrinol 71, 141-153. doi:10.1016/0303-7207(90)90251-3
Tascher, G., Burban, A., Camus, S. et al. (2019). In-depth proteome analysis highlights HepaRG Cells as a versatile cell system surrogate for primary human hepatocytes. Cells 8, 192. doi:10.3390/cells8020192
Tinwell, H., Rouquié, D., Schorsch, F. et al. (2014). Liver tumor formation in female rat induced by fluopyram is mediated by CAR/PXR nuclear receptor activation. Regul Toxicol Pharmacol 70, 648-658. doi:10.1016/j.yrtph.2014.09.011
Toda, S., Yonernitsu, N., Hikichi, Y. et al. (1992). Differentiation of human thyroid follicle cells from normal subjects and Basedow’s disease in three-dimensional collagen gel culture. Pathol Res Pract 188, 874-882. doi:10.1016/S0344-0338(11)80247-5
Toda, S., Aoki, S., Uchihashi, K. et al. (2011). Culture models for studying thyroid biology and disorders. ISRN Endocrinol 2011, 275782. doi:10.5402/2011/275782
Tomida, T., Okamura, H., Satsukawa, M. et al. (2015). Multiparametric assay using HepaRG cells for predicting drug-induced liver injury. Toxicol Lett 236, 16-24. doi:10.1016/j.toxlet.2015.04.014
Uhlén, M., Fagerberg, L., Hallström, B. M. et al. (2015). Proteomics. Tissue-based map of the human proteome. Science 347, 1260419. doi:10.1126/science.1260419
Underhill, G. H. and Khetani, S. R. (2018). Bioengineered liver models for drug testing and cell differentiation studies. Cell Mol Gastroenterol Hepatol 5, 426-439.e1. doi:10.1016/j.jcmgh.2017.11.012
Van der Spek, A. H., Fliers, E. and Boelen, A. (2017). The classic pathways of thyroid hormone metabolism. Mol Cell Endocrinol 458, 29-38. doi:10.1016/j.mce.2017.01.025
Vargas-Uricoechea, H., Bonelo-Perdomo, A. and Sierra-Torres, C. H. (2014). Effects of thyroid hormones on the heart. Clin Investig Arterioscler 26, 296-309. doi:10.1016/j.arteri.2014.07.003
Vickers, A. E. M., Heale, J., Sinclair, J. R. et al. (2012). Thyroid organotypic rat and human cultures used to investigate drug effects on thyroid function, hormone synthesis and release pathways. Toxicol Appl Pharmacol 260, 81-88. doi:10.1016/j.taap.2012.01.029
Visser, W. E., Friesema, E. C. H. and Visser, T. J. (2011). Minireview: Thyroid hormone transporters: The knowns and the unknowns. Mol Endocrinol 25, 1-14. doi:10.1210/me.2010-0095
Wang, J., Hallinger, D. R., Murr, A. S. et al. (2019). High-throughput screening and chemotype-enrichment analysis of ToxCast phase II chemicals evaluated for human sodium-iodide symporter (NIS) inhibition. Environ Int 126, 377-386. doi:10.1016/j.envint.2019.02.024
Wang, Z.-Y., Li, W.-J., Li, Q.-G. et al. (2019). A DMSO-free hepatocyte maturation medium accelerates hepatic differentiation of HepaRG cells in vitro. Biomed Pharmacother 116, 109010. doi:10.1016/j.biopha.2019.109010
Willoughby, K. A., Mcandrews, M. P. and Rovet, J. (2013). Effects of early thyroid hormone deficiency on children’s autobiographical memory performance. J Int Neuropsychol Soc 19, 419-429. doi:10.1017/S1355617712001488
Xie, W., Barwick, J. L., Downes, M. et al. (2000). Humanized xenobiotic response in mice expressing nuclear receptor SXR. Nature 406, 435-439. doi:10.1038/35019116
Yokoyama, Y., Sasaki, Y., Terasaki, N. et al. (2018). Comparison of drug metabolism and its related hepatotoxic effects in HepaRG, cryopreserved human hepatocytes, and HepG2 cell cultures. Biol Pharm Bull 41, 722-732. doi:10.1248/bpb.b17-00913
Zellmer, S., Schmidt-Heck, W., Godoy, P. et al. (2010). Transcription factors ETF, E2F, and SP-1 are involved in cytokine-independent proliferation of murine hepatocytes. Hepatology 52, 2127-2136. doi:10.1002/hep.23930