Dog as the experimental model: Laboratory uses of dogs in the United States
Main Article Content
Abstract
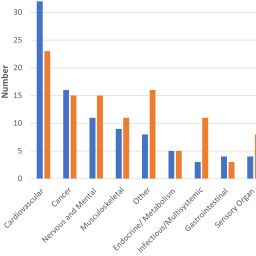
Dogs are the experimental model in many types of biomedical research. Each year, hundreds of publications report the use of dogs in invasive biomedical procedures, often without sufficient explanation of the purpose and justification for selecting dog as the experimental model. The European Union requires detailed reporting of animal use that includes research purpose, but animal use reporting in the United States, overseen by the USDA, does not require this information. The ability to replace dogs with alternative models begins by understanding how they are used. Therefore, this study was undertaken to investigate the types of invasive biomedical procedures that dogs are subjected to in US laboratories. Well-defined sets of research publications and grants were accessed to obtain information on the types of biomedical research using dogs. USDA databases provided additional information. An ontology to categorize biomedical research uses of dogs identified the most common as translational studies for cardiovascular, cancer, nervous/mental, and musculoskeletal disorders. Information typically reported for experimental animals was sometimes missing or incomplete in publications, including the number, source, fate, species justification, and pain management of dogs, suggesting that many journals have not adopted the ARRIVE guidelines on animal use reporting. It was not possible to identify the research purpose for all dogs used by US institutions because (a) not all dog use is published and (b) animal research purpose is not required reporting in the US. These findings should be informative to future initiatives to replace, reduce, and refine the use of dogs in research.
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.
Articles are distributed under the terms of the Creative Commons Attribution 4.0 International license (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution and reproduction in any medium, provided the original work is appropriately cited (CC-BY). Copyright on any article in ALTEX is retained by the author(s).
Barthelemy, I., Hitte, C. and Tiret, L. (2019). The dog model in the spotlight: Legacy of a trustful cooperation. J Neuromuscul Dis 6, 421-451. doi:10.3233/JND-190394
Bertrand, H., Sandersen, C. and Flecknell, P. A. (2018). Reported analgesic and anaesthetic administration to non-human primates undergoing experimental surgical procedure: 2010-2015. J Med Primatol, online ahead of print. doi:10.1111/jmp.12346
Box, R. J. and Spielmann, H. (2005). Use of the dog as non-rodent test species in the safety testing schedule associated with the registration of crop and plant protection products (pesticides): Present status. Arch Toxicol 79, 615-626. doi:10.1007/s00204-005-0678-0
Carbone, L. (2012). Pain management standards in the eighth edition of the Guide for the Care and Use of Laboratory Animals. J Am Assoc Lab Anim Sci 51, 322-328.
Carbone, L. and Austin, J. (2016). Pain and laboratory animals: Publication practices for better data reproducibility and better animal welfare. PLoS One 11, e0155001. doi:10.1371/journal.pone.0155001
Cashman, T. J., Josowitz, R., Johnson, B. V. et al. (2016). Human engineered cardiac tissues created using induced pluripotent stem cells reveal functional characteristics of BRAF-mediated hypertrophic cardiomyopathy. PLoS One 11, e0146697. doi:10.1371/journal.pone.0146697
Coulter, C. A., Flecknell, P. A. and Richardson, C. A. (2009). Reported analgesic administration to rabbits, pigs, sheep, dogs and non-human primates undergoing experimental surgical procedures. Lab Anim 43, 232-238. doi:10.1258/la.2008.008021
EC – European Commission (2012). Commission Implementing Decision of 14 November 2012 Establishing a Common Format for the Submission of the Information Pursuant to Directive 2010/63/EU of the European Parliament and of the Council on the Protection of Animals Used for Scientific Purposes. https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:02012D0707-20140115
EC (2020a). Report from the Commission to the European Parliament and the Council. 2019 Report on the Statistics on the Use of Animals for Scientific Purposes in the Member States of the European Union in 2015-2017, COM/2020/16 final. https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A52020DC0016&qid=1625787931541
EC (2020b). Commission Staff Working Document – 2019 Report on the Statistics on the Use of Animals for Scientific Purposes in the Member States of the European Union in 2015-2017. SWD(2020) 10 final. https://eur-lex.europa.eu/legal-content/EN/TXT/?qid=1581689520921&uri=CELEX:52020SC0010
Fenwick, N., Duffus, S. E. and Griffin, G. (2014). Pain management for animals used in science: Views of scientists and veterinarians in Canada. Animals 4, 494-514. doi:10.3390/ani4030494
Fleischer, S., Sharkey, M., Mealey, K. et al. (2008). Pharmacogenetic and metabolic differences between dog breeds: Their impact on canine medicine and the use of the dog as a preclinical animal model. AAPS J 10, 110-119. doi:10.1208/s12248-008-9011-1
Funakoshi, S. and Yoshida, Y. (2021). Recent progress of iPSC technology in cardiac diseases. Arch Toxicol 95, 3633-3650. doi:10.1007/s00204-021-03172-3
Gähwiler, E., Motta, S. E., Martin, M. et al. (2021). Human iPSCs and genome editing technologies for precision cardiovascular tissue engineering. Front Cell Dev Biol 9, 639699. doi:10.3389/fcell.2021.639699
Guittin, P. and Decelle, T. (2002). Future improvements and implementation of animal care practices within the animal testing regulatory environment. ILAR J 43, Suppl 1, S80-S84, doi:10.1093/ilar.43.Suppl_1.S80
Hanton, G. and Rabemampianina, Y. (2006). The electrocardiogram of the Beagle dog: Reference values and effect of sex, genetic strain, body position and heart rate. Lab Anim 40, 123-136. doi:10.1258/002367706776319088
Harper, E. J., Hackett, R. M., Wilkinson, J. et al. (2003). Age-related variations in hematologic and plasma biochemical test results in Beagles and Labrador Retrievers. J Am Vet Med Assoc 223, 1436-1442. doi:10.2460/javma.2003.223.1436
Hasiwa, N., Bailey, J., Clausing, P. et al. (2011). t4 workshop report: Critical evaluation of the use of dogs in biomedical research and testing in Europe. ALTEX 28, 326-340. doi:10.14573/altex.2011.4.326
Herrmann, K. and Flecknell, P. (2019). Retrospective review of anesthetic and analgesic regimens used in animal research proposals. ALTEX 36, 65-80. doi:10.14573/altex.1804011
Ikeda-Douglas, C. J., de Rivera, C. and Milgram, N. W. (2005). Pharmaceutical and other commercial uses of the dog model. Prog Neuropsychopharmacol Biol Psychiatry 29, 355-360. doi:10.1016/j.pnpbp.2004.12.001
Ishikawa, K. (ed.) (2018). Experimental Models of Cardiovascular Diseases. Methods in Molecular Biology 1816. New York, USA: Humana Press. doi:10.1007/978-1-4939-8597-5
Kringe, L., Sena, E. S., Motschall, E. et al. (2020). Quality and validity of large animal experiments in stroke: A systematic review. J Cereb Blood Flow Metab 40, 2152-2164. doi:10.1177/0271678X20931062
Lee, J. G., Sung, Y. H. and Baek, I. J. (2018). Generation of genetically-engineered animals using engineered endonucleases. Arch Pharm Res 41, 885-897. doi:10.1007/s12272-018-1037-z
Li, R. A., Keung, W., Cashman, T. J. et al. (2018). Bioengineering an electro-mechanically functional miniature ventricular heart chamber from human pluripotent stem cells. Biomaterials 163, 116-127. doi:10.1016/j.biomaterials.2018.02.024
Maddah, M., Mandegar, M. A., Dame, K. et al. (2020). Quantifying drug-induced structural toxicity in hepatocytes and cardiomyocytes derived from hiPSCs using a deep learning method. J Pharmacol Toxicol Methods 105, 106895. doi:10.1016/j.vascn.2020.106895
Milan, D. J. and MacRae, C. A. (2005). Animal models for arrhythmias. Cardiovasc Res 67, 426-437. doi:10.1016/j.cardiores.2005.06.012
NASEM – National Academies of Sciences, Engineering, and Medicine (2020). Necessity, Use, and Care of Laboratory Dogs at the U.S. Department of Veterans Affairs. Washington, DC, USA: The National Academies Press. doi:10.17226/25772
NIH OLAW – Office of Laboratory Animal Welfare (2015). Public Health Service Policy on Humane Care and Use of Laboratory Animals. NIH Publication No. 15-8013.
NRC – National Research Council (1994). Laboratory Animal Management: Dogs. Washington, DC, USA: The National Academies Press. doi:10.17226/2120
NRC (2009a). Scientific and Humane Issues in the Use of Random Source Dogs and Cats in Research. Washington, DC, USA: The National Academies Press. doi:10.17226/12641
NRC (2009b). Recognition and Alleviation of Pain in Laboratory Animals. Washington, DC, USA: The National Academies Press. doi:10.17226/12526
NRC (2011). Guide for the Care and Use of Laboratory Animals. 8th edition. Washington, DC, USA: The National Academies Press. doi:10.17226/12910
Oh, J. G. and Ishikawa, K. (2018). Experimental models of cardiovascular diseases: Overview. In K. Ishikawa (ed.), Experimental Models of Cardiovascular Diseases (3-14). Methods Mol Biol 1816. New York, USA: Humana Press. doi:10.1007/978-1-4939-8597-5_1
Percie du Sert, N., Ahluwalia, A., Alam, S. et al. (2020a). Reporting animal research: Explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol 18, e3000411. doi:10.1371/journal.pbio.3000411
Percie du Sert, N., Hurst, V., Ahluwalia, A. et al. (2020b). The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol 18, e3000410. doi:10.1371/journal.pbio.3000410
Powers, J. C. and Recchia, F. (2018). Canine model of pacing-induced heart failure. In K. Ishikawa (ed.), Experimental Models of Cardiovascular Diseases (309-325). Methods Mol Biol 1816. New York, NY, USA: Humana Press. doi:10.1007/978-1-4939-8597-5_24
Ribeiro, A., Guth, B. D., Engwall, M. et al. (2019). Considerations for an in vitro, cell-based testing platform for detection of drug-induced inotropic effects in early drug development. Part 2: Designing and fabricating microsystems for assaying cardiac contractility with physiological relevance using human iPSC-cardiomyocytes. Front Pharmacol 10, 934. doi:10.3389/fphar.2019.00934
Savoji, H., Mohammadi, M. H., Rafatian, N. et al. (2019). Cardiovascular disease models: A game changing paradigm in drug discovery and screening. Biomaterials 198, 3-26. doi:10.1016/j.biomaterials.2018.09.036
Shen, Y.-T., Chen, L., Testani, J. M. et al. (2017). Animal models for cardiovascular research. In P. M. Conn (ed.), Animal Models for the Study of Human Disease (2nd edition) (147-174). London, UK: Academic Press. doi:10.1016/B978-0-12-809468-6.00006-1
Simon, L. R. and Masters, K. S. (2020). Disease-inspired tissue engineering: Investigation of cardiovascular pathologies. ACS Biomater Sci Eng 6, 2518-2532. doi:10.1021/acsbiomaterials.9b01067
Switonski, M. (2020). Impact of gene therapy for canine monogenic diseases on the progress of preclinical studies. J Appl Genet 61, 179-186. doi:10.1007/s13353-020-00554-8
Turnbull, I. C., Karakikes, I., Serrao, G. W. et al. (2014). Advancing functional engineered cardiac tissues toward a preclinical model of human myocardium. FASEB J 28, 644-654. doi:10.1096/fj.13-228007
Turnbull, I. C., Mayourian, J., Murphy, J. F. et al. (2018). Cardiac tissue engineering models of inherited and acquired cardiomyopathies. In K. Ishikawa (ed.), Experimental Models of Cardiovascular Diseases (145-159). Methods Mol Biol 1816. New York, USA: Humana Press. doi:10.1007/978-1-4939-8597-5_11
Uhlig, C., Krause, H., Koch, T. et al. (2015). Anesthesia and monitoring in small laboratory mammals used in anesthesiology, respiratory and critical care research: A systematic review on the current reporting in top-10 impact factor ranked journals. PLoS One 10, e0134205. doi:10.1371/journal.pone.0134205
van der Naald, M., Wenker, S., Doevendans, P. A. et al. (2020). Publication rate in preclinical research: A plea for preregistration. BMJ Open Sci 4, e100051. doi:10.1136/bmjos-2019-100051
Wilson, S., Nagel, S. J., Frizon, L. A. et al. (2020). The hemisection approach in large animal models of spinal cord injury: Overview of methods and applications. J Invest Surg 33, 240-251. doi:10.1080/08941939.2018.1492048
Yoo, S., Aistrup, G., Shiferaw, Y. et al. (2018). Oxidative stress creates a unique, CaMKII-mediated substrate for atrial fibrillation in heart failure. JCI Insight 3, e120728. doi:10.1172/jci.insight.120728