Development of a network of carcinogenicity adverse outcome pathways and its employment as an evidence framework for safety assessment
Main Article Content
Abstract
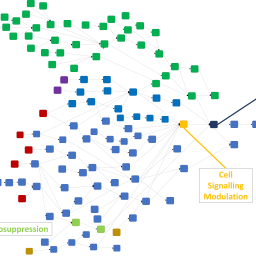
The traditional paradigm for safety assessment of chemicals for their carcinogenic potential to humans relies heavily on a battery of well-established genotoxicity tests, usually followed up by long-term, high-dose rodent studies. There are a variety of problems with this approach, not least that the rodent may not always be the best model to predict toxicity in humans. Consequently, new approach methodologies (NAMs) are being developed to replace or enhance predictions coming from the existing assays. However, a combination of the data arising from NAMs is likely to be required to improve upon the current paradigm, and consequently a framework is needed to combine evidence in a meaningful way. Adverse outcome pathways (AOPs) represent an ideal construct on which to organize this evidence. In this work, a data structure outlined previously was used to capture AOPs and evidence relating to carcinogenicity. Knowledge held within the predictive system Derek Nexus was extracted, built upon, and arranged into a coherent network containing 37 AOPs. 60 assays and 351 in silico alerts were then associated with KEs in this network, and it was brought to life by associating data and contextualizing evidence and predictions for over 13,400 compounds. Initial investigations into using the network to view knowledge and reason between evidence in different ways were made. Organizing knowledge and evidence in this way provides a flexible framework on which to carry out more consistent and meaningful carcinogenicity safety assessments in many different contexts.
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.
Articles are distributed under the terms of the Creative Commons Attribution 4.0 International license (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution and reproduction in any medium, provided the original work is appropriately cited (CC-BY). Copyright on any article in ALTEX is retained by the author(s).
Amberg, A., Harvey, J. S., Czich, A. et al. (2015). Do carboxylic/sulfonic acid halides really present a mutagenic and carcinogenic risk as impurities in final drug products? Org Process Res Dev 19, 1495-1506. doi:10.1021/acs.oprd.5b00106
Ankley, G. T., Bennett, R. S., Erickson, R. J. et al. (2010). Adverse outcome pathways: A conceptual framework to support ecotoxicology research and risk assessment. Environ Toxicol Chem 29, 730-741. doi:10.1002/etc.34
Arnesdotter, E., Spinu, N., Firman, J. et al. (2021). Derivation, characterisation and analysis of an adverse outcome pathway network for human hepatotoxicity. Toxicology 459, 152856. doi:10.1016/j.tox.2021.152856
Ball, T., Barber, C. G., Cayley, A. et al. (2021). Beyond adverse outcome pathways: Making toxicity predictions from event networks, SAR models, data and knowledge. Toxicol Res 10, 102-122. doi:10.1093/toxres/tfaa099
Bayly, A. C., Roberts, R. A. and Dive, C. (1994). Suppression of liver cell apoptosis in vitro by the non-genotoxic hepatocarcinogen and peroxisome proliferator nafenopin. J Cell Biol 125, 197-203. doi:10.1083/jcb.125.1.197
Berry, C. (2017). The failure of rodent carcinogenesis as a model for Man. Toxicol Res 7, 553-557. doi:10.1039/c7tx00283a
Boobis, A. R., Cohen, S. M., Dellarco, V. L. et al. (2016). Classification schemes for carcinogenicity based on hazard-identification have become outmoded and serve neither science nor society. Regul Toxicol Pharmacol 82, 158-166. doi:10.1016/j.yrtph.2016.10.014
Bryce, S. M., Bernacki, D. T., Bemis, J. C. et al. (2016). Genotoxic mode of action predictions from a multiplexed flow cytometric assay and a machine learning approach. Environ Mol Mutagen 57, 171-189. doi:10.1002/em.21996
Cohen, S. M. (2004). Human carcinogenic risk evaluation: An alternative approach to the two-year rodent bioassay. Toxicol Sci 80, 225-229. doi:10.1093/toxsci/kfh159
Cohen, S. M., Boobis, A. R., Dellarco, V. L. et al. (2019). Chemical carcinogenicity revisited 3: Risk assessment of carcinogenic potential based on the current state of knowledge of carcinogenesis in humans. Regul Toxicol Pharmacol 103, 100-105. doi:10.1016/j.yrtph.2019.01.017
Columbano, A., Ledda-Columbano, G. M., Pibiri, M. et al. (2005). Gadd45beta is induced through a CAR-dependent, TNF-independent pathway in murine liver hyperplasia. Hepatology 42, 1118-1126. doi:10.1002/hep.20883
Cunningham, M. L., Collins, B. J., Hejtmancik, M. R. et al. (2010). Effects of the PPARα agonist and widely used antihyperlipidemic drug gemfibrozil on hepatic toxicity and lipid metabolism. PPAR Res 2010, 681963. doi:10.1155/2010/681963
Doe, J. E., Boobis, A. R., Dellarco, V. et al. (2019). Chemical carcinogenicity revisited 2: Current knowledge of carcinogenesis shows that categorization as a carcinogen or non-carcinogen is not scientifically credible. Regul Toxicol Pharmacol 103, 124-129. doi:10.1016/j.yrtph.2019.01.024
Dix, D. J., Houck, K. A., Martin, M. T. et al. (2007). The ToxCast program for prioritizing toxicity testing of environmental chemicals. Toxicol Sci 95, 5-12. doi:10.1093/toxsci/kfl103
Guyton, K. Z., Kyle, A. D., Aubrecht, J. et al. (2009). Improving prediction of chemical carcinogenicity by considering multiple mechanisms and applying toxicogenomic approaches. Mutat Res 681, 230-240. doi:10.1016/j.mrrev.2008.10.001
Helm, J. S. and Rudel, R. A. (2020). Adverse outcome pathways for ionizing radiation and breast cancer involve direct and indirect DNA damage, oxidative stress, inflammation, genomic instability, and interaction with hormonal regulation of the breast. Arch Toxicol 94, 1511-1549. doi:10.1007/s00204-020-02752-z
Hendriks, G., Atallah, M., Morolli, B. et al. (2012). The ToxTracker assay: Novel GFP reporter systems that provide mechanistic insight into the genotoxic properties of chemicals. Toxicol Sci 125, 285-298. doi:10.1093/toxsci/kfr281
Heusinkveld, H., Braakhuis, H., Gommans, R. et al. (2020). Towards a mechanism-based approach for the prediction of nongenotoxic carcinogenic potential of agrochemicals. Crit Rev Toxicol 50, 725-739. doi:10.1080/10408444.2020.1841732
Hill, III T. and Conolly, R. B. (2019). Development of a novel AOP for Cyp2F2-mediated lung cancer in mice. Toxicol Sci 172, 1-10. doi:10.1093/toxsci/kfz185
Howes, A. J., Chan, V. S. and Caldwell, J. (1990). Structure-specificity of the genotoxicity of some naturally occurring alkenylbenzenes determined by the unscheduled DNA synthesis assay in rat hepatocytes. Food Chem Toxicol 28, 537-542. doi:10.1016/0278-6915(90)90152-d
Huang, W., Zhang, J., Washington, M. et al. (2005). Xenobiotic stress induces hepatomegaly and liver tumors via the nuclear receptor constitutive androstane receptor. Mol Endocrinol 19, 1646-1653. doi:10.1210/me.2004-0520
IARC (1987). International Agency for Research on Cancer (IARC) – Summaries & Evaluations – Safrole, Isosafrole, and Dihydrosafrole. INCHEM Suppl. 7, 71. https://inchem.org/documents/iarc/vol10/safrole.html
IARC (2012). Pharmaceuticals – A review of human carcinogens. IARC Monogr Eval Carcinog Risks Hum 100A, 175. https://inchem.org/documents/iarc/iarcmono/v100aiarc.pdf
IARC (2019). IARC Monographs on the Identification of Carcinogenic Hazards to Humans. https://monographs.iarc.who.int/wp-content/uploads/2019/07/Preamble-2019.pdf
ICH S1B (1997). ICH Harmonised Tripartite Guideline: Testing for Carcinogenicity of Pharmaceuticals S1B. International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. https://database.ich.org/sites/default/files/S1B%20Guideline.pdf
ICH S1B(R1) (2021). ICH Harmonised Guideline: Addendum to the Guideline on Testing for Carcinogenicity of Pharmaceuticals. International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. https://database.ich.org/sites/default/files/ICH_S1BR1_Step2_DraftGuideline_2021_0510.pdf.
ICH S2(R1) (2011). ICH Harmonised Tripartite Guideline: Guidance on Genotoxicity Testing and Data Interpretation for Pharmaceuticals Intended for Human Use S2(R1). International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. https://database.ich.org/sites/default/files/S2%28R1%29%20Guideline.pdf
Ives, C., Campia, I., Wang, R. L. et al. (2017). Creating a structured AOP knowledgebase via ontology-based annotations. Appl In Vitro Toxicol 3, 298-311. doi:10.1089/aivt.2017.0017
Jacobs, M. N., Colacci, A., Louekari, K. et al. (2016). International regulatory needs for development of an IATA for non-genotoxic carcinogenic chemical substances. ALTEX 33, 359-392. doi:10.14573/altex.1601201
Jacobs, M. N., Colacci, A., Corvi, R. et al. (2020). Chemical carcinogen safety testing: OECD expert group international consensus on the development of an integrated approach for the testing and assessment of chemical non-genotoxic carcinogens. Arch Toxicol 94, 2899-2923. doi:10.1007/s00204-020-02784-5
Jayasekara, P. S., Skanchy, S. K., Kim, M. T. et al. (2021). Assessing the impact of expert knowledge on ICH M7 (Q)SAR predictions. Is expert review still needed? Regul Toxicol Pharmacol 125, 105006. doi:10.1016/j.yrtph.2021.105006
Johansson, H. K. L., Damdimopoulou, P., van Duursen, M. B. M. et al. (2020). Putative adverse outcome pathways for female reproductive disorders to improve testing and regulation of chemicals. Arch Toxicol 94, 3359-3379. doi:10.1007/s00204-020-02834-y
Klaunig, J. E., Babich, M. A., Baetcke, K. P. et al. (2003). PPARalpha agonist-induced rodent tumors: Modes of action and human relevance. Crit Rev Toxicol 33, 655-780. doi:10.1080/713608372
Knapen, D., Angrish, M. M., Fortin, M. C. et al. (2018). Adverse outcome pathway networks I: Development and applications. Environ Toxicol Chem 37, 1723-1733. doi:10.1002/etc.4125
Kodama, S. and Negishi, M. (2011). Pregnane X receptor PXR activates the GADD45beta gene, eliciting the p38 MAPK signal and cell migration. J Biol Chem 286, 3570-3578. doi:10.1074/jbc.M110.179812
Lichtermann, D., Ekelund, J., Pukkala, E. et al. (2001). Incidence of cancer among persons with schizophrenia and their relatives. Arch Gen Psychiatry 58, 573-578. doi:10.1001/archpsyc.58.6.573
Lien, S. C., Wei, S. Y., Chang, S. F. et al. (2013). Activation of PPAR-α induces cell cycle arrest and inhibits transforming growth factor-β1 induction of smooth muscle cell phenotype in 10T1/2 mesenchymal cells. Cell Signal 25, 1252-1263. doi:10.1016/j.cellsig.2013.01.021
Lynch, A. M., Eastmond, D., Elhajouji, A. et al. (2019). Targets and mechanisms of chemically induced aneuploidy. Part 1 of the report of the 2017 IWGT workgroup on assessing the risk of aneugens for carcinogenesis and hereditary diseases. Mutat Res 847, 403025. doi:10.1016/j.mrgentox.2019.02.006
Nymark, P., Karlsson, H. L., Halappanavar, S. et al. (2021). Adverse outcome pathway development for assessment of lung carcinogenicity by nanoparticles. Front Toxicol 3, 653386. doi:10.3389/ftox.2021.653386
OECD (2017). Guidance Document for the Use of Adverse Outcome Pathways in Developing Integrated Approaches to Testing and Assessment (IATA). OECD Series on Testing and Assessment, No. 260. OECD Publishing, Paris. doi:10.1787/44bb06c1-en
OECD (2018). Users’ Handbook supplement to the Guidance Document for developing and assessing Adverse Outcome Pathways. OECD Series on Adverse Outcome Pathways, No. 1. OECD Publishing, Paris. doi:10.1787/5jlv1m9d1g32-en
OECD (2021). Guideline No. 497: Defined Approaches on Skin Sensitisation. OECD Guidelines for the Testing of Chemicals, Section 4. OECD Publishing, Paris. doi:10.1787/b92879a4-en
Pollesch, N. L., Villeneuve, D. L. and O’Brien, J. M. (2019). Extracting and benchmarking emerging adverse outcome pathway knowledge. Toxicol Sci 168, 349-364. doi:10.1093/toxsci/kfz006
Robbins, D., Cherian, M., Wu, J. et al. (2016). Human pregnane X receptor compromises the function of p53 and promotes malignant transformation. Cell Death Discov 2, 16023. doi:10.1038/cddiscovery.2016.23
Sasaki, J. C., Allemang, A., Bryce, S. M. et al. (2020). Application of the adverse outcome pathway framework to genotoxic modes of action. Environ Mol Mutagen 61, 114-134. doi:10.1002/em.22339
Sewell, F., Gellatly, N., Beaumont, M. et al. (2018). The future trajectory of adverse outcome pathways: A commentary. Arch Toxicol 92, 1657-1661. doi:10.1007/s00204-018-2183-2
Sistare, F. D., Morton, D., Alden, C. et al. (2011). An analysis of pharmaceutical experience with decades of rat carcinogenicity testing: Support for a proposal to modify current regulatory guidelines. Toxicol Pathol 39, 716-744. doi:10.1177/0192623311406935
Smith, M. T., Guyton, K. Z., Gibbons, C. F. et al. (2016). Key characteristics of carcinogens as a basis for organizing data on mechanisms of carcinogenesis. Environ Health Perspect 124, 713-721. doi:10.1289/ehp.1509912
Spinu, N., Cronin, M. T. D., Enoch, S. J. et al. (2020). Quantitative adverse outcome pathway (qAOP) models for toxicity prediction. Arch Toxicol 94, 1497-1510. doi:10.1007/s00204-020-02774-7
Stalford, S. A., Cayley, A. N. and de Oliveira, A. A. F. (2021). Employing an adverse outcome pathway framework for weight-of-evidence assessment with application to the ICH S1B guidance addendum. Reg Toxicol Pharmacol 127, 105071. doi:10.1016/j.yrtph.2021.105071
Swanson, A. B., Chambliss, D. D., Blomquist, J. C. et al. (1979). The mutagenicities of safrole, estragole, eugenol, trans-anethole, and some of their known or possible metabolites for Salmonella typhimurium mutants, Mutat Res 60, 143-153. doi:10.1016/0027-5107(79)90178-7
Tian, J., Huang, H., Hoffman, B. et al. (2011). Gadd45β is an inducible coactivator of transcription that facilitates rapid liver growth in mice. J Clin Invest 121, 4491-4502. doi:10.1172/JCI38760
Wang, P. S., Walker, A. M., Tsuang, M. T. et al. (2002). Dopamine antagonists and the development of breast cancer. Arch Gen Psychiatry 59, 1147-1154. doi:10.1001/archpsyc.59.12.1147
Wolf, D. C., Cohen, S. M., Boobis, A. R. et al. (2019) Chemical carcinogenicity revisited 1: A unified theory of carcinogenicity based on contemporary knowledge. Regul Toxicol Pharmacol 103, 86-92. doi:10.1016/j.yrtph.2019.01.021