Identifying candidate reference chemicals for in vitro testing of the retinoid pathway for predictive developmental toxicity
Main Article Content
Abstract
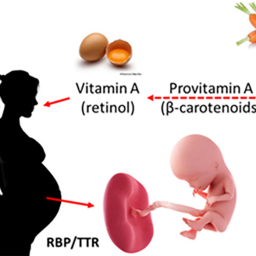
Evaluating chemicals for potential in vivo toxicity based on their in vitro bioactivity profile is an important step toward animal- free testing. A compendium of reference chemicals and data describing their bioactivity on specific molecular targets, cellular pathways, and biological processes is needed to bolster confidence in the predictive value of in vitro hazard detection. Endogenous signaling by all-trans retinoic acid (ATRA) is an important pathway in developmental processes and toxicities. Employing data extraction methods and advanced literature extraction tools, we assembled a set of candidate reference chemicals with demonstrated activity on ten protein family targets in the retinoid system. The compendium was culled from Protein Data Bank, ChEMBL, ToxCast/Tox21, and the biomedical literature in PubMed. Finally, we performed a case study on one chemical in our collection, citral, an inhibitor of endogenous ATRA production, to determine whether the literature supports an adverse outcome pathway explaining the compound’s developmental toxicity initiated by disruption of the retinoid pathway. We also deliver an updated Abstract Sifter tool populated with these reference compounds and complex search terms designed to query the literature for the downstream consequences to support concordance with targeted retinoid pathway disruption.
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.
Articles are distributed under the terms of the Creative Commons Attribution 4.0 International license (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution and reproduction in any medium, provided the original work is appropriately cited (CC-BY). Copyright on any article in ALTEX is retained by the author(s).
Abramovici, A., Liban, E., Ben-David, E. et al. (1973). The ultrastructure of striated muscle in malformed chick limb induced by citral. Virchows Arch B Cell Pathol 14, 127-134. doi:10.1007/bf02889182
Abramovici, A., Rachmuth-Forschmidt, P., Liban, E. et al. (1980). Experimental limb dysmorphogenesis as a model of chemical injury response in undifferentiated embryonic tissues: A light and electron microscopical study. J Pathol 131, 289-308. doi:10.1002/path.1711310402
Agarwal, C., Chandraratna, R. A., Johnson, A. T. et al. (1996). AGN193109 is a highly effective antagonist of retinoid action in human ectocervical epithelial cells. J Biol Chem 271, 12209-12212. doi:10.1074/jbc.271.21.12209
Allali-Hassani, A., Peralba, J. M., Martras, S. et al. (1998). Retinoids, omega-hydroxyfatty acids and cytotoxic aldehydes as physiological substrates, and H2-receptor antagonists as pharmacological inhibitors, of human class IV alcohol dehydrogenase. FEBS Lett 426, 362-366. doi:10.1016/s0014-5793(98)00374-3
Allen, E. M., Anderson, D. G., Florang, V. R. et al. (2010). Relative inhibitory potency of molinate and metabolites with aldehyde dehydrogenase 2: Implications for the mechanism of enzyme inhibition. Chem Res Toxicol 23, 1843-1850. doi:10.1021/tx100317q
Anonymous (2004). Tamibarotene: AM 80, retinobenzoic acid, tamibaro. Drugs R D 5, 359-362. doi:10.2165/00126839-200405060-00010
Attene-Ramos, M. S., Miller, N., Huang, R. et al. (2013). TheTox21 robotic platform for the assessment of environmental chemicals – From vision to reality. Drug Discov Today 18, 716-723. doi:10.1016/j.drudis.2013.05.015
Baker, N. C. and Hemminger, B. M. (2010). Mining connections between chemicals, proteins, and diseases extracted from Medline annotations. J Biomed Inform 43, 510-519. doi:10.1016/j.jbi.2010.03.008
Baker, N., Knudsen, T. and Williams, A. (2017). Abstract Sifter: A comprehensive front-end system to PubMed. F1000Res 6, 2164. doi:10.12688/f1000research.12865.1
Balmer, J. E. and Blomhoff, R. (2002). Gene expression regulation by retinoic acid. J Lipid Res 43, 1773-1808. doi:10.1194/jlr.r100015-jlr200
Bell, R. G. and Smith, H. W. (1949). Preliminary report on clinical trials of antabuse. Can Med Assoc J 60, 286-288.
Berman, H., Henrick, K. and Nakamura, H. (2003). Announcing the worldwide Protein Data Bank. Nat Struct Biol 10, 980. doi:10.1038/nsb1203-980
Berry, D. C., O’Byrne, S. M., Vreeland, A. C. et al. (2012). Cross talk between signaling and vitamin a transport by the retinol-binding protein receptor STRA6. Mol Cell Biol 32, 3164-3175. doi:10.1128/mcb.00505-12
Blume-Peytavi, U., Fowler, J., Kemeny, L. et al. (2020). Long-term safety and efficacy of trifarotene 50 µg/g cream, a first-in-class RAR-γ selective topical retinoid, in patients with moderate facial and truncal acne. J Eur Acad Dermatol Venereol 34, 166-173. doi:10.1111/jdv.15794
Boerman, M. H. and Napoli, J. L. (1995). Characterization of a microsomal retinol dehydrogenase: A short-chain alcohol dehydrogenase with integral and peripheral membrane forms that interacts with holo-CRBP (type I). Biochemistry 34, 7027-7037. doi:10.1021/bi00021a014
Breen, C. J., Martin, D. S., Ma, H. et al. (2015). Production of functional human vitamin A transporter/RBP receptor (STRA6) for structure determination. PLoS One 10, e0122293. doi:10.1371/journal.pone.0122293
Brtko, J. and Dvorak, Z. (2020). Natural and synthetic retinoid X receptor ligands and their role in selected nuclear receptor action. Biochimie 179, 157-168. doi:10.1016/j.biochi.2020.09.027
Bruno, R. D. and Njar, V. C. (2007). Targeting cytochrome P450 enzymes: A new approach in anti-cancer drug development. Bioorg Med Chem 15, 5047-5060. doi:10.1016/j.bmc.2007.05.046
Buttrick, B. R. (2013). Characterization of selective and potent inhibitors of the human retinoic acid hydroxylases CYP26A1 and CYP26B1. MSc thesis, University of Washington. https://bit.ly/3X23tOZ
Cao, S., Wang, G., Ge, F. et al. (2019). Gossypol inhibits 5α-reductase 1 and 3α-hydroxysteroid dehydrogenase: Its possible use for the treatment of prostate cancer. Fitoterapia 133, 102-108. doi:10.1016/j.fitote.2018.12.024
Chaudhuri, B. N., Kleywegt, G. J., Broutin-L’Hermite, I. et al. (1999). Structures of cellular retinoic acid binding proteins I and II in complex with synthetic retinoids. Acta Crystallogr D Biol Crystallogr 55, 1850-1857. doi:10.1107/s0907444999011026
Chen, C. H., Lin, K. D., Ke, L. Y. et al. (2019). O-GlcNAcylation disrupts STRA6-retinol signals in kidneys of diabetes. Biochim Biophys Acta Gen Subj 1863, 1059-1069. doi:10.1016/j.bbagen.2019.03.014
Chen, H., Chidboy, M. A. and Robinson, J. F. (2020). Retinoids and developmental neurotoxicity: Utilizing toxicogenomics to enhance adverse outcome pathways and testing strategies. Reprod Toxicol 96, 102-113. doi:10.1016/j.reprotox.2020.06.007
Chen, R., Chen, F., Han, J. et al. (2001). Effects of selective rar or/and rxr retinoids on the proliferation and differentiation of nb4 cells and their mechanisms [Article in Chinese]. Zhonghua Xue Ye Xue Za Zhi 22, 256-259.
Chen, W. S., Bohlken, D. P. and Plapp, B. V. (1981). Inactivation of liver alcohol dehydrogenases and inhibition of ethanol metabolism by ambivalent active-site-directed reagents. J Med Chem 24, 190-193. doi:10.1021/jm00134a012
Chen, Y. and Reese, D. H. (2013). A screen for disruptors of the retinol (vitamin A) signaling pathway. Birth Defects Res B Dev Reprod Toxicol 98, 276-282. doi:10.1002/bdrb.21062
Chen, Y. and Reese, D. H. (2016). Disruption of retinol (vitamin A) signaling by phthalate esters: SAR and mechanism studies. PLoS One 11, e0161167. doi:10.1371/journal.pone.0161167
Chen, Y., Clarke, O. B., Kim, J. et al. (2016a). Structure of the stra6 receptor for retinol uptake. Science 353, aad8266. doi:10.1126/science.aad8266
Chen, Y., Sakamuru, S., Huang, R. et al. (2016b). Identification of compounds that modulate retinol signaling using a cell-based qHTS assay. Toxicol In Vitro 32, 287-296. doi:10.1016/j.tiv.2016.01.011
Chen, Y., Zhu, J. Y., Hong, K. H. et al. (2018). Structural basis of ALDH1A2 inhibition by irreversible and reversible small molecule inhibitors. ACS Chem Biol 13, 582-590. doi:10.1021/acschembio.7b00685
Cioffi, C. L., Dobri, N., Freeman, E. E. et al. (2014). Design, synthesis, and evaluation of nonretinoid retinol binding protein 4 antagonists for the potential treatment of atrophic age-related macular degeneration and Stargardt disease. J Med Chem 57, 7731-7757. doi:10.1021/jm5010013
Collins, M. D., Eckhoff, C., Chahoud, I. et al. (1992). 4-Methylpyrazole partially ameliorated the teratogenicity of retinol and reduced the metabolic formation of all-trans-retinoic acid in the mouse. Arch Toxicol 66, 652-659. doi:10.1007/bf01981505
Connor, M. J. and Smit, M. H. (1987). Terminal-group oxidation of retinol by mouse epidermis. Inhibition in vitro and in vivo. Biochem J 244, 489-492. doi:10.1042/bj2440489
Connor, M. J. (1988). Oxidation of retinol to retinoic acid as a requirement for biological activity in mouse epidermis. Cancer Res 48, 7038-7040.
Damdimopoulou, P., Chiang, C. and Flaws, J. A. (2019). Retinoic acid signaling in ovarian folliculogenesis and steroidogenesis. Reprod Toxicol 87, 32-41. doi:10.1016/j.reprotox.2019.04.007
Donovan, M., Olofsson, B., Gustafson, A. L. et al. (1995). The cellular retinoic acid binding proteins. J Steroid Biochem Mol Biol 53, 459-465. doi:10.1016/0960-0760(95)00092-e
Duester, G. (1991). A hypothetical mechanism for fetal alcohol syndrome involving ethanol inhibition of retinoic acid synthesis at the alcohol dehydrogenase step. Alcohol Clin Exp Res 15, 568-572. doi:10.1111/j.1530-0277.1991.tb00562.x
Duester, G. (2008). Retinoic acid synthesis and signaling during early organogenesis. Cell 134, 921-931. doi:10.1016/j.cell.2008.09.002
Elmazar, M. M., Reichert, U., Shroot, B. et al. (1996). Pattern of retinoid-induced teratogenic effects: Possible relationship with relative selectivity for nuclear retinoid receptors RAR alpha, RAR beta, and RAR gamma. Teratology 53, 158-167. doi:10.1002/(sici)1096-9926(199603)53:3<158::aid-tera3>3.0.co;2-0
Favorskaya, I., Kainov, Y., Chemeris, G. et al. (2014). Expression and clinical significance of CRABP1 and CRABP2 in non-small cell lung cancer. Tumour Biol 35, 10295-10300. doi:10.1007/s13277-014-2348-4
Filer, D. L., Kothiya, P., Setzer, R. W. et al. (2017). tcpl: The toxcast pipeline for high-throughput screening data. Bioinformatics 33, 618-620. doi:10.1093/bioinformatics/btw680
Finulli, M. and Magistretti, M. (1961). [Antabuse-like toxic manifestations in workmen employed in the manufacture of a synthetic anticryptogamic: T.M.T.D. (tetramethylthiuram disulfide) [Article in Italian]. Med Lav 52, 132-137.
Fogh, K., Voorhees, J. J. and Astrom, A. (1993). Expression, purification, and binding properties of human cellular retinoic acid-binding protein type I and type II. Arch Biochem Biophys 300, 751-755. doi:10.1006/abbi.1993.1104
Foti, R. S., Isoherranen, N., Zelter, A. et al. (2016a). Identification of tazarotenic acid as the first xenobiotic substrate of human retinoic acid hydroxylase CYP26A1 and CYP26B1. J Pharmacol Exp Ther 357, 281-292. doi:10.1124/jpet.116.232637
Foti, R. S., Diaz, P. and Douguet, D. (2016b). Comparison of the ligand binding site of CYP2C8 with CYP26A1 and CYP26B1: A structural basis for the identification of new inhibitors of the retinoic acid hydroxylases. J Enzyme Inhib Med Chem 31, 148-161. doi:10.1080/14756366.2016.1193734
Galdones, E. and Hales, B. F. (2008). Retinoic acid receptor gamma-induced misregulation of chondrogenesis in the murine limb bud in vitro. Toxicol Sci 106, 223-232. doi:10.1093/toxsci/kfn169
Galli, A., Pinaire, J., Fischer, M. et al. (2001). The transcriptional and DNA binding activity of peroxisome proliferator-activated receptor alpha is inhibited by ethanol metabolism. A novel mechanism for the development of ethanol-induced fatty liver. J Biol Chem 276, 68-75. doi:10.1074/jbc.m008791200
Garnier, R., Chataigner, D. and Efthymiou, M. L. (1992). Skin and eye burns, painful abdomen syndrome, antabuse effect, and cytolytic hepatitis in workers exposed to dimethylformamide [Article in French]. J Toxicol Clin Exp 12, 227-237.
Gaulton, A., Hersey, A., Nowotka, M. et al. (2017). The ChEMBL database in 2017. Nucleic Acids Res 45, D945-D954. doi:10.1093/nar/gkw1074
Gaworski, C. L., Vollmuth, T. A., York, R. G. et al. (1992). Developmental toxicity evaluation of inhaled citral in Sprague-Dawley rats. Food Chem Toxicol 30, 269-275. doi:10.1016/0278-6915(92)90003-4
Germain, P., Gaudon, C., Pogenberg, V. et al. (2009). Differential action on coregulator interaction defines inverse retinoid agonists and neutral antagonists. Chem Biol 16, 479-489. doi:10.1016/j.chembiol.2009.03.008
Grignard, E., Hakansson, H. and Munn, S. (2020). Regulatory needs and activities to address the retinoid system in the context of endocrine disruption: The European viewpoint. Reprod Toxicol 93, 250-258. doi:10.1016/j.reprotox.2020.03.002
Helvig, C., Taimi, M., Cameron, D. et al. (2011). Functional properties and substrate characterization of human CYP26A1, CYP26B1, and CYP26C1 expressed by recombinant baculovirus in insect cells. J Pharmacol Toxicol Methods 64, 258-263. doi:10.1016/j.vascn.2011.08.005
Hu, W., Verschraegen, C. F., Wu, W. G. et al. (2002). Activity of ALRT 1550, a new retinoid, with interferon-gamma on ovarian cancer cell lines. Int J Gynecol Cancer 12, 202-207. doi:10.1046/j.1525-1438.2002.01084.x
Huddle, B. C., Grimley, E., Buchman, C. D. et al. (2018). Structure-based optimization of a novel class of aldehyde dehydrogenase 1A (ALDH1A) subfamily-selective inhibitors as potential adjuncts to ovarian cancer chemotherapy. J Med Chem 61, 8754-8773. doi:10.1021/acs.jmedchem.8b00930.s002
Hussain, R. M., Gregori, N. Z., Ciulla, T. A. et al. (2018). Pharmacotherapy of retinal disease with visual cycle modulators. Expert Opin Pharmacother 19, 471-481. doi:10.1080/14656566.2018.1448060
Jacobsen, E. and Larsen, V. (1949). Site of the formation of acetaldehyde after ingestion of antabuse (tetraethylthiuramdisulphide) and alcohol. Acta Pharmacol Toxicol (Copenh) 5, 285-291. doi:10.1111/j.1600-0773.1949.tb03393.x
Jin, N., Zhu, X., Cheng, F. et al. (2018). Disulfiram/copper targets stem cell-like ALDH+ population of multiple myeloma by inhibition of ALDH1A1 and hedgehog pathway. J Cell Biochem 119, 6882-6893. doi:10.1002/jcb.26885
Johnson, A. T., Klein, E. S., Gillett, S. J. et al. (1995). Synthesis and characterization of a highly potent and effective antagonist of retinoic acid receptors. J Med Chem 38, 4764-4767. doi:10.1021/jm00024a003
Ju, J., Wang, N., Wang, J. et al. (2018). 4-Amino-2-trifluoromethyl-phenyl retinate inhibits proliferation, invasion, and migration of breast cancer cells by independently regulating CRABP2 and FABP5. Drug Des Devel Ther 12, 997-1008. doi:10.2147/dddt.s151029
Judson, R. S., Houck, K. A., Kavlock, R. J. et al. (2010). In vitro screening of environmental chemicals for targeted testing prioritization: The ToxCast project. Environ Health Perspect 118, 485-492. doi:10.1289/ehp.0901392
Judson, R. S., Thomas, R. S., Baker, N. et al. (2019). Workflow for defining reference chemicals for assessing performance of in vitro assays. ALTEX 36, 261-276. doi:10.14573/altex.1809281
Kagechika, H., Kawachi, E., Hashimoto, Y. et al. (1988). Retinobenzoic acids. 1. Structure-activity relationships of aromatic amides with retinoidal activity. J Med Chem 31, 2182-2192. doi:10.1021/jm00119a021
Kaminskaia, Z. A. (1949). Primenenie citralia pri glaukome [Application of citral in glaucoma]. Sov Med 13, 37.
Kar, A. B., Jehan, Q., Kamboj, V. P. et al. (1966). Effect of N,N'-bis(dichloroacetyl)-1,8-octamethylenediamine on the chemical composition of the rat seminiferous tubules. Int J Fertil 11, 291-296.
Kast, R. E. and Belda-Iniesta, C. (2009). Suppressing glioblastoma stem cell function by aldehyde dehydrogenase inhibition with chloramphenicol or disulfiram as a new treatment adjunct: An hypothesis. Curr Stem Cell Res Ther 4, 314-317. doi:10.2174/157488809789649241
Kawaguchi, R. and Sun, H. (2010). Techniques to study specific cell-surface receptor-mediated cellular vitamin A uptake. Methods Mol Biol 652, 341-361. doi:10.1007/978-1-60327-325-1_20
Kelly, M. and von Lintig, J. (2015). STRA6: Role in cellular retinol uptake and efflux. Hepatobiliary Surg Nutr 4, 229-242. doi:10.3978/j.issn.2304-3881.2015.01.12
Kikonyogo, A., Abriola, D. P., Dryjanski, M. et al. (1999). Mechanism of inhibition of aldehyde dehydrogenase by citral, a retinoid antagonist. Eur J Biochem 262, 704-712. doi:10.1046/j.1432-1327.1999.00415.x
Kim, S., Chen, J., Cheng, T. et al. (2019). PubChem 2019 update: Improved access to chemical data. Nucleic Acids Res 47, D1102-D1109. doi:10.1093/nar/gky1033
Kim, Y. J., Kim, J. Y., Lee, N. et al. (2017). Disulfiram suppresses cancer stem-like properties and STAT3 signaling in triple-negative breast cancer cells. Biochem Biophys Res Commun 486, 1069-1076. doi:10.1016/j.bbrc.2017.03.164
Klaholz, B. P., Renaud, J. P., Mitschler, A. et al. (1998). Conformational adaptation of agonists to the human nuclear receptor RAR gamma. Nat Struct Biol 5, 199-202. doi:10.1038/nsb0398-199
Knudsen, T. B., Pierro, J. D. and Baker, N. C. (2021). Retinoid signaling in skeletal development: Scoping the system for predictive toxicology. Reprod Toxicol 99, 109-130. doi:10.1016/j.reprotox.2020.10.014
Kochhar, D. M. (1973). Limb development in mouse embryos. I. Analysis of teratogenic effects of retinoic acid. Teratology 7, 289-295. doi:10.1002/tera.1420070310
Kochhar, D. M., Jiang, H., Penner, J. D. et al. (1998). The use of a retinoid receptor antagonist in a new model to study vitamin A-dependent developmental events. Int J Dev Biol 42, 601-608.
Koppaka, V., Thompson, D. C., Chen, Y. et al. (2012). Aldehyde dehydrogenase inhibitors: A comprehensive review of the pharmacology, mechanism of action, substrate specificity, and clinical application. Pharmacol Rev 64, 520-539. doi:10.1124/pr.111.005538
Kumar, S., Sandell, L. L., Trainor, P. A. et al. (2012). Alcohol and aldehyde dehydrogenases: Retinoid metabolic effects in mouse knockout models. Biochimica et Biophysica Acta 1821, 198-205. doi:10.1016/j.bbalip.2011.04.004
Lampen, A., Meyer, S., Arnhold, T. et al. (2000). Metabolism of vitamin A and its active metabolite all-trans-retinoic acid in small intestinal enterocytes. J Pharmacol Exp Ther 295, 979-985.
Lee, G. S., Kochhar, D. M. and Collins, M. D. (2004). Retinoid-induced limb malformations. Curr Pharm Des 10, 2657-2699. doi:10.2174/1381612043383728
Lee, S. A., Yang, K. J. Z., Brun, P. J. et al. (2020). Retinol-binding protein 2 (RBP2) binds monoacylglycerols and modulates gut endocrine signaling and body weight. Sci Adv 6, eaay8937. doi:10.1126/sciadv.aay8937
Lemaire, G., Balaguer, P., Michel, S. et al. (2005). Activation of retinoic acid receptor-dependent transcription by organochlorine pesticides. Toxicol Appl Pharmacol 202, 38-49. doi:10.1016/j.taap.2004.06.004
Li, D., Wang, M., Cheng, S. et al. (2017). CYP1A1 based on metabolism of xenobiotics by cytochrome P450 regulates chicken male germ cell differentiation. In Vitro Cell Dev Biol Anim 53, 293-303. doi:10.1007/s11626-016-0108-z
Li, Z., Yao, S. J., Alini, M. et al. (2011). The role of retinoic acid receptor inhibitor LE135 on the osteochondral differentiation of human bone marrow mesenchymal stem cells. J Cell Biochem 112, 963-970. doi:10.1002/jcb.23013
Lim, W., Ham, J., Park, S. et al. (2019). Gossypol induces disruption of spermatogenesis and steroidogenesis in male mice. J Agric Food Chem 67, 2075-2085. doi:10.1021/acs.jafc.8b06946
Liu, P., Brown, S., Goktug, T. et al. (2012). Cytotoxic effect of disulfiram/copper on human glioblastoma cell lines and ALDH-positive cancer-stem-like cells. Br J Cancer 107, 1488-1497. doi:10.1038/bjc.2012.442
Liu, R. Z., Garcia, E., Glubrecht, D. D. et al. (2015). CRABP1 is associated with a poor prognosis in breast cancer: Adding to the complexity of breast cancer cell response to retinoic acid. Mol Cancer 14, 129. doi:10.1186/s12943-015-0380-7
Lu, C., Li, X., Ren, Y. et al. (2021). Disulfiram: A novel repurposed drug for cancer therapy. Cancer Chemother Pharmacol 87, 159-172. doi:10.1007/s00280-020-04216-8
Maguire, M., Larsen, M. C., Vezina, C. M. et al. (2020). Cyp1b1 directs Srebp-mediated cholesterol and retinoid synthesis in perinatal liver; association with retinoic acid activity during fetal development. PLoS One 15, e0228436. doi:10.1371/journal.pone.0228436
Mao, B., Wu, C., Zheng, W. et al. (2018). Methoxychlor and its metabolite HPTE inhibit rat neurosteroidogenic 3α-hydroxysteroid dehydrogenase and retinol dehydrogenase 2. Neurosci Lett 684, 169-174. doi:10.1016/j.neulet.2018.08.008
Mark, M., Ghyselinck, N. B. and Chambon, P. (2009). Function of retinoic acid receptors during embryonic development. Nucl Recept Signal 7, e002. doi:10.1621/nrs.07002
Metzler, M. A. and Sandell, L. L. (2016). Enzymatic metabolism of vitamin A in developing vertebrate embryos. Nutrients 8, 812. doi:10.3390/nu8120812
Mey, J., Babiuk, R. P., Clugston, R. et al. (2003). Retinal dehydrogenase-2 is inhibited by compounds that induce congenital diaphragmatic hernias in rodents. Am J Pathol 162, 673-679. doi:10.1016/s0002-9440(10)63861-8
Molotkov, A. and Duester, G. (2002). Retinol/ethanol drug interaction during acute alcohol intoxication in mice involves inhibition of retinol metabolism to retinoic acid by alcohol dehydrogenase. J Biol Chem 277, 22553-22557. doi:10.1074/jbc.m201603200
Momma, K., Ando, M. and Takao, A. (1990). Fetal cardiac morphology of tetralogy of Fallot with absent pulmonary valve in the rat. Circulation 82, 1343-1351. doi:10.1161/01.cir.82.4.1343
Moretti, A., Li, J., Donini, S. et al. (2016). Crystal structure of human aldehyde dehydrogenase 1A3 complexed with NAD+ and retinoic acid. Sci Rep 6, 35710. doi:10.1038/srep35710
Morgan, C. A. and Hurley, T. D. (2015). Characterization of two distinct structural classes of selective aldehyde dehydrogenase 1A1 inhibitors. J Med Chem 58, 1964-1975. doi:10.1021/jm501900s
Morgan, C. A., Parajuli, B., Buchman, C. D. et al. (2015). N,N-diethylaminobenzaldehyde (DEAB) as a substrate and mechanism-based inhibitor for human ALDH isoenzymes. Chem Biol Interact 234, 18-28. doi:10.1016/j.cbi.2014.12.008
Mujawar, I., Sabatino, M., Ray Mitchell, S. et al. (2014). A 12-year comparison of students’ perspectives on diversity at a Jesuit medical school. Med Educ Online 19, 23401. doi:10.3402/meo.v19.23401
Napoli, J. L. (2012). Physiological insights into all-trans-retinoic acid biosynthesis. Biochim Biophys Acta 1821, 152-167. doi:10.1016/j.bbalip.2011.05.004
Napoli, J. L. (2016). Functions of intracellular retinoid binding-proteins. Subcell Biochem 81, 21-76. doi:10.1007/978-94-024-0945-1_2
Napoli, J. L. (2017). Cellular retinoid binding-proteins, CRBP, CRABP, FABP5: Effects on retinoid metabolism, function and related diseases. Pharmacol Ther 173, 19-33. doi:10.1016/j.pharmthera.2017.01.004
Napoli, J. L. (2020). Post-natal all-trans-retinoic acid biosynthesis. Methods Enzymol 637, 27-54. doi:10.1016/bs.mie.2020.02.003
Niederreither, K., Vermot, J., Schuhbaur, B. et al. (2002). Embryonic retinoic acid synthesis is required for forelimb growth and anteroposterior patterning in the mouse. Development 129, 3563-3574. doi:10.1242/dev.129.15.3563
Nogueira, A. C., Carvalho, R. R., Souza, C. A. et al. (1995). Study on the embryofeto-toxicity of citral in the rat. Toxicology 96, 105-113. doi:10.1016/0300-483x(94)02915-h
Noy, N. (2016). Vitamin A transport and cell signaling by the retinol-binding protein receptor STRA6. Subcell Biochem 81, 77-93. doi:10.1007/978-94-024-0945-1_3
OECD (2014). Guidance Document on Standardised Test Guidelines for Evaluating Chemicals for Endocrine Disruption. OECD Publishing, Paris. doi:10.1787/9789264221413-en
OECD (2021). Detailed Review Paper on the Retinoid System. Series on Testing and Assessment, No. 343. OECD Publishing, Paris. https://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=ENV-CBC-MONO(2021)20%20&doclanguage=en
Oster, G., Salgo, M. P. and Taleporos, P. (1974). Embryocidal action of a bis(dichloroacetyl)-diamine: An oral abortifacient for rats. Am J Obstet Gynecol 119, 583-588. doi:10.1016/0002-9378(74)90117-3
Pasutto, F., Sticht, H., Hammersen, G. et al. (2007). Mutations in STRA6 cause a broad spectrum of malformations including anophthalmia, congenital heart defects, diaphragmatic hernia, alveolar capillary dysplasia, lung hypoplasia, and mental retardation. Am J Hum Genet 80, 550-560. doi:10.1086/512203
Persson, B., Kallberg, Y., Bray, J. E. et al. (2009). The SDR (short-chain dehydrogenase/reductase and related enzymes) nomenclature initiative. Chem Biol Interact 178, 94-98. doi:10.1016/j.cbi.2008.10.040
Pignatello, M. A., Kauffman, F. C. and Levin, A. A. (1997). Multiple factors contribute to the toxicity of the aromatic retinoid, TTNPB (Ro 13-7410): Binding affinities and disposition. Toxicol Appl Pharmacol 142, 319-327. doi:10.1006/taap.1996.8047
Plouvier, B., Lemoine, X., De Coninck, P. et al. (1982). Antabuse effect during the administration of a topical drug based on monosulfiram [Article in French]. Nouv Presse Med 11, 3209.
Quattrini, L., Gelardi, E. L. M., Coviello, V. et al. (2020). Imidazo[1,2-a]pyridine derivatives as aldehyde dehydrogenase inhibitors: Novel chemotypes to target glioblastoma stem cells. J Med Chem 63, 4603-4616. doi:10.1021/acs.jmedchem.9b01910
Quistad, G. B., Sparks, S. E. and Casida, J. E. (1994). Aldehyde dehydrogenase of mice inhibited by thiocarbamate herbicides. Life Sci 55, 1537-1544. doi:10.1016/0024-3205(94)00314-9
Racz, B., Varadi, A., Kong, J. et al. (2018). A non-retinoid antagonist of retinol-binding protein 4 rescues phenotype in a model of Stargardt disease without inhibiting the visual cycle. J Biol Chem 293, 11574-11588. doi:10.1074/jbc.ra118.002062
Raha, D., Wilson, T. R., Peng, J. et al. (2014). The cancer stem cell marker aldehyde dehydrogenase is required to maintain a drug-tolerant tumor cell subpopulation. Cancer Res 74, 3579-3590. doi:10.1158/0008-5472.can-13-3456
Reynier, M. (1969). Pyrazole inhibition and kinetic studies of ethanol and retinol oxidation catalyzed by rat liver alcohol dehydrogenase. Acta Chem Scand 23, 1119-1129. doi:10.3891/acta.chem.scand.23-1119
Richard, A. M., Judson, R. S., Houck, K. A. et al. (2016). ToxCast chemical landscape: Paving the road to 21st century toxicology. Chem Res Toxicol 29, 1225-1251. doi:10.1021/acs.chemrestox.6b00135
Rowbotham, S. E., Illingworth, N. A., Daly, A. K. et al. (2010). Role of UDP-glucuronosyltransferase isoforms in 13-cis retinoic acid metabolism in humans. Drug Metab Dispos 38, 1211-1217. doi:10.1124/dmd.109.031625
Ruberte, E., Friederich, V., Morriss-Kay, G. et al. (1992). Differential distribution patterns of CRABP I and CRABP II transcripts during mouse embryogenesis. Development 115, 973-987. doi:10.1242/dev.115.4.973
Schindler, J. F., Berst, K. B. and Plapp, B. V. (1998). Inhibition of human alcohol dehydrogenases by formamides. J Med Chem 41, 1696-1701. doi:10.1021/jm9707380
Scialli, A. R., Daston, G., Chen, C. et al. (2018). Rethinking developmental toxicity testing: Evolution or revolution? Birth Defects Res 110, 840-850. doi:10.1002/bdr2.1212
Shao, Z. M., Dawson, M. I., Li, X. S. et al. (1995). P53 independent G0/G1 arrest and apoptosis induced by a novel retinoid in human breast cancer cells. Oncogene 11, 493-504.
Sharma, V., Sharma, A., Kumar, V. et al. (2009). Disulfiram-like reaction with ornidazole. J Postgrad Med 55, 292-293. doi:10.4103/0022-3859.58940
Shimomura, T., Kawakami, M., Okuda, H. et al. (2015). Retinoic acid regulates Lhx8 expression via FGF-8b to the upper jaw development of chick embryo. J Biosci Bioeng 119, 260-266. doi:10.1016/j.jbiosc.2014.08.010
Silvaroli, J. A., Widjaja-Adhi, M. A. K., Trischman, T. et al. (2019). Abnormal cannabidiol modulates vitamin A metabolism by acting as a competitive inhibitor of CRBP1. ACS Chem Biol 14, 434-448. doi:10.1021/acschembio.8b01070
Silvaroli, J. A., Plau, J., Adams, C. H. et al. (2021). Molecular basis for the interaction of cellular retinol binding protein 2 (CRBP2) with nonretinoid ligands. J Lipid Res 62, 100054. doi:10.1016/j.jlr.2021.100054
Singh, A. K. and Dominic, C. J. (1995). Testicular toxicity of WIN 18446 in the laboratory mouse. Reprod Toxicol 9, 475-481. doi:10.1016/0890-6238(95)00039-d
Song, S. and Xu, X. C. (2001). Effect of benzo[a]pyrene diol epoxide on expression of retinoic acid receptor-beta in immortalized esophageal epithelial cells and esophageal cancer cells. Biochem Biophys Res Commun 281, 872-877. doi:10.1006/bbrc.2001.4433
Song, Y., Hui, J. N., Fu, K. K. et al. (2004). Control of retinoic acid synthesis and FGF expression in the nasal pit is required to pattern the craniofacial skeleton. Dev Biol 276, 313-329. doi:10.1016/j.ydbio.2004.08.035
Su, Y., Li, H., Chen, X. et al. (2018). Ziram inhibits rat neurosteroidogenic 5α-reductase 1 and 3α-hydroxysteroid dehydrogenase. Toxicol Mech Methods 28, 38-44. doi:10.1080/15376516.2017.1355950
Sun, X., Zhang, Z., Ning, H. et al. (2017). Sitagliptin down-regulates retinol-binding protein 4 and reduces insulin resistance in gestational diabetes mellitus: A randomized and double-blind trial. Metab Brain Dis 32, 773-778. doi:10.1007/s11011-017-9958-7
Takahashi, M., Yang, X. J., Lavery, T. T. et al. (2002). Gene expression profiling of favorable histology Wilms tumors and its correlation with clinical features. Cancer Res 62, 6598-6605.
Tan, J., Thiboutot, D., Popp, G. et al. (2019). Randomized phase 3 evaluation of trifarotene 50 µg/g cream treatment of moderate facial and truncal acne. J Am Acad Dermatol 80, 1691-1699. doi:10.1016/j.jaad.2019.02.044
Tanaka, M., Tamura, K. and Ide, H. (1996). Citral, an inhibitor of retinoic acid synthesis, modifies chick limb development. Dev Biol 175, 239-247. doi:10.1006/dbio.1996.0111
Thacher, S. M., Nagpal, S., Klein, E. S. et al. (1999). Cell type and gene-specific activity of the retinoid inverse agonist AGN 193109: Divergent effects from agonist at retinoic acid receptor gamma in human keratinocytes. Cell Growth Differ 10, 255-262.
Thanacoody, R. H., Gilfillan, C., Bradberry, S. M. et al. (2016). Management of poisoning with ethylene glycol and methanol in the UK: A prospective study conducted by the national poisons information service (NPIS). Clin Toxicol (Phila) 54, 134-140. doi:10.3109/15563650.2015.1116044
Thatcher, J. E., Buttrick, B., Shaffer, S. A. et al. (2011). Substrate specificity and ligand interactions of CYP26A1, the human liver retinoic acid hydroxylase. Mol Pharmacol 80, 228-239. doi:10.1124/mol.111.072413
Thomas, M. L., de Antueno, R., Coyle, K. M. et al. (2016). Citral reduces breast tumor growth by inhibiting the cancer stem cell marker ALDH1A3. Mol Oncol 10, 1485-1496. doi:10.1016/j.molonc.2016.08.004
Thoreau, E., Arlabosse, J. M., Bouix-Peter, C. et al. (2018). Structure-based design of trifarotene (CD5789), a potent and selective RARγ agonist for the treatment of acne. Bioorg Med Chem Lett 28, 1736-1741. doi:10.1016/j.bmcl.2018.04.036
USEPA (2018). Strategic Plan to Promote the Development and Implementation of Alternative Test Methods within the TSCA Program. U.S. Environmental Protection Agency, Office of Chemical Safety and Pollution Prevention, Washington, DC. https://www.epa.gov/sites/default/files/2018-06/documents/epa_alt_strat_plan_6-20-18_clean_final.pdf
Vandersea, M. W., Fleming, P., McCarthy, R. A. et al. (1998). Fin duplications and deletions induced by disruption of retinoic acid signaling. Dev Genes Evol 208, 61-68. doi:10.1007/s004270050155
Venkataramaiah, T. H. and Plapp, B. V. (2003). Formamides mimic aldehydes and inhibit liver alcohol dehydrogenases and ethanol metabolism. J Biol Chem 278, 36699-36706. doi:10.1074/jbc.m305419200
Vermot, J., Schuhbaur, B., Le Mouellic, H. et al. (2005). Retinaldehyde dehydrogenase 2 and Hoxc8 are required in the murine brachial spinal cord for the specification of Lim1+ motoneurons and the correct distribution of Islet1+ motoneurons. Development 132, 1611-1621. doi:10.1242/dev.01718
Villeneuve, D. L., Crump, D., Garcia-Reyero, N. et al. (2014). Adverse outcome pathway (AOP) development I: Strategies and principles. Toxicol Sci 142, 312-320. doi:10.1093/toxsci/kfu199
Wang, B., Yan, Y., Zhou, J. et al. (2013). A novel all-trans retinoid acid derivatives inhibits the migration of breast cancer cell lines MDA-MB-231 via myosin light chain kinase involving p38-MAPK pathway. Biomed Pharmacother 67, 357-362. doi:10.1016/j.biopha.2013.03.016
Wang, C., Kane, M. A. and Napoli, J. L. (2011). Multiple retinol and retinal dehydrogenases catalyze all-trans-retinoic acid biosynthesis in astrocytes. J Biol Chem 286, 6542-6553. doi:10.1074/jbc.m110.198382
Wang, N. N., Wang, L. H., Li, Y. et al. (2018). Targeting ALDH2 with disulfiram/copper reverses the resistance of cancer cells to microtubule inhibitors. Exp Cell Res 362, 72-82. doi:10.1016/j.yexcr.2017.11.004
Wang, Y., Connors, R., Fan, P. et al. (2014). Structure-assisted discovery of the first non-retinoid ligands for retinol-binding protein 4. Bioorg Med Chem Lett 24, 2885-2891. doi:10.1016/j.bmcl.2014.04.089
Wang, Y., Sun, J., Chen, L. et al. (2017). Effects of resveratrol on rat neurosteroid synthetic enzymes. Fitoterapia 122, 61-66. doi:10.1016/j.fitote.2017.08.005
Wei, L. N. (2016). Cellular retinoic acid binding proteins: Genomic and non-genomic functions and their regulation. Subcell Biochem 81, 163-178. doi:10.1007/978-94-024-0945-1_6
Wiley, M. J. (1983). The pathogenesis of retinoic acid-induced vertebral abnormalities in golden Syrian hamster fetuses. Teratology 28, 341-353. doi:10.1002/tera.1420280306
Williams, A. J., Grulke, C. M., Edwards, J. et al. (2017). The CompTox chemistry dashboard: A community data resource for environmental chemistry. J Cheminform 9, 61. doi:10.1186/s13321-017-0247-6
Xie, P. T. and Hurley, T. D. (1999). Methionine-141 directly influences the binding of 4-methylpyrazole in human sigma sigma alcohol dehydrogenase. Protein Sci 8, 2639-2644. doi:10.1110/ps.8.12.2639
Xu, J., Zhang, M., Zhang, X. et al. (2018). Contribution of hepatic retinaldehyde dehydrogenase induction to impairment of glucose metabolism by high-fat-diet feeding in C57BL/6J mice. Basic Clin Pharmacol Toxicol 123, 539-548. doi:10.1111/bcpt.13039
Yasgar, A., Titus, S. A., Wang, Y. et al. (2017). A high-content assay enables the automated screening and identification of small molecules with specific ALDH1A1-inhibitory activity. PLoS One 12, e0170937. doi:10.1371/journal.pone.0170937
Yashiro, K., Zhao, X., Uehara, M. et al. (2004). Regulation of retinoic acid distribution is required for proximodistal patterning and outgrowth of the developing mouse limb. Dev Cell 6, 411-422. doi:10.1016/s1534-5807(04)00062-0
Yu, J., Gonzalez, S., Martinez, L. et al. (2003). Effects of retinoic acid on the neural crest-controlled organs of fetal rats. Pediatr Surg Int 19, 355-358. doi:10.1007/s00383-003-1010-9
Zhang, J., Pu, K., Bai, S. et al. (2020). The anti-alcohol dependency drug disulfiram inhibits the viability and progression of gastric cancer cells by regulating the Wnt and NF-κB pathways. J Int Med Res 48, 300060520925996. doi:10.1177/0300060520925996
Zhang, L., Nadzan, A. M., Heyman, R. A. et al. (1996). Discovery of novel retinoic acid receptor agonists having potent antiproliferative activity in cervical cancer cells. J Med Chem 39, 2659-2663. doi:10.1021/jm960285j
Zhong, M., Kawaguchi, R., Costabile, B. et al. (2020). Regulatory mechanism for the transmembrane receptor that mediates bidirectional vitamin A transport. Proc Natl Acad Sci U S A 117, 9857-9864. doi:10.1073/pnas.1918540117