How to formulate hypotheses and IATAs to support grouping and read-across of nanoforms
Main Article Content
Abstract
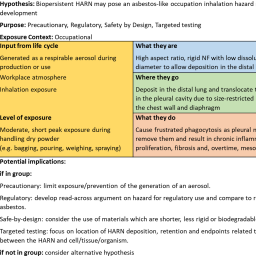
Manufacturing and functionalizing materials at the nanoscale has led to the generation of a whole array of nanoforms (NFs) of substances varying in size, morphology, and surface characteristics. Due to financial, time, and ethical considerations, testing every unique NF for adverse effects is virtually impossible. Use of hypothesis-driven grouping and read-across approaches, as supported by the GRACIOUS Framework, represents a promising alternative to case-by-case testing that will make the risk assessment process more efficient. Through application of appropriate grouping hypotheses, the Framework facilitates the assessment of similarity between NFs, thereby supporting grouping and read-across of information, minimizing the need for new testing, and aligning with the 3R principles of replacement, reduction, and refinement of animals in toxicology studies. For each grouping hypothesis an integrated approach to testing and assessment (IATA) guides the user in data gathering and acquisition to test the hypothesis, following a structured format to facilitate efficient decision-making. Here we present the template used to generate the GRACIOUS grouping hypotheses encompassing information relevant to “Lifecycle, environmental release, and human exposure”, “What they are: physicochemical characteristics”, “Where they go: environmental fate, uptake, and toxicokinetics”, and “What they do: human and environmental toxicity”. A summary of the template-derived hypotheses focusing on human health is provided, along with an overview of the IATAs generated by the GRACIOUS project. We discuss the application and flexibility of the template, providing the opportunity to expand the application of grouping and read-across in a logical, evidence-based manner to a wider range of NFs and substances.
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.
Articles are distributed under the terms of the Creative Commons Attribution 4.0 International license (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution and reproduction in any medium, provided the original work is appropriately cited (CC-BY). Copyright on any article in ALTEX is retained by the author(s).
Arts, J. H. E., Hadi, M., Keene, A. M. et al. (2014). A critical appraisal of existing concepts for the grouping of nanomaterials. Regul Toxicol Pharmacol 70, 492-506. doi:10.1016/j.yrtph.2014.07.025
Arts, J. H., Irfan, M. A., Keene, A. M. et al. (2016). Case studies putting the decision-making framework for the grouping and testing of nanomaterials (DF4nanoGrouping) into practice. Regul Toxicol Pharmacol 76, 234-261. doi:10.1016/j.yrtph.2015.11.020
Aschberger, K., Christensen, F. M., Rasmussen, K. et al. (2016). Feasibility and challenges of human health risk assessment for engineered nanomaterials. In B. Xing, C. D. Vecitis and N. Senesi (eds.), Engineered Nanoparticles and the Environment: Biophysicochemical Processes and Toxicity. Wiley Online Library. doi:10.1002/9781119275855.ch21
Ball, N., Cronin, M. T., Shen, J. et al. (2016). Toward good read-across practice (GRAP) guidance. ALTEX 33, 149-166. doi:10.14573/altex.1601251
Bernstein, D. M., Mast, R., Anderson, R. et al. (1994). An experimental approach to the evaluation of the biopersistence of respirable synthetic fibers and minerals. Environ Health Perspect 102, Suppl 5, 15-18. doi:10.1289/ehp.94102s515
Bernstein, D. M., Morscheidt, C., de Meringo, A. et al. (1997). The biopersistence of fibres following inhalation and intratracheal instillation exposure. Ann Occup Hyg 41, 224-230.
Bottero, J.-Y., Auffan, M., Borschnek, D. et al. (2015). Nanotechnology, global development in the frame of environmental risk forecasting. A necessity of interdisciplinary researches. CR Geosci 347, 35-42. doi:10.1016/j.crte.2014.10.004
Bove, P., Malvindi, M. A., Kote, S. S. et al. (2017). Dissolution test for risk assessment of nanoparticles: A pilot study. Nanoscale 9, 6315-6326. doi:10.1039/c6nr08131b
Braakhuis, H. M., Murphy, F., Ma-Hock, L. et al. (2021). An integrated approach to testing and assessment to support grouping and read-across of nanomaterials after inhalation exposure. Appl In Vitro Toxicol 7, 112-128. doi:10.1089/aivt.2021.0009
Broaddus, V. C., Everitt, J. I., Black, B. et al. (2011). Non-neoplastic and neoplastic pleural endpoints following fiber exposure. J Toxicol Environ Health B Crit Rev 14, 153-178. doi:10.1080/10937404.2011.556049
Burden, N., Aschberger, K., Chaudhry, Q. et al. (2017). The 3Rs as a framework to support a 21st century approach for nanosafety assessment. Nano Today 12, 10-13. doi:10.1016/j.nantod.2016.06.007
Carnovale, C., Guarnieri, D., Di Cristo, L. et al. (2021). Biotransformation of silver nanoparticles into oro-gastrointestinal tract by integrated in vitro testing assay: Generation of exposure-dependent physical descriptors for nanomaterial grouping. Nanomaterials 11, 1587. doi:10.3390/nano11061587
Cogliano, V. J., Baan, R. A., Straif, K. et al. (2008). Use of mechanistic data in IARC evaluations. Environ Mol Mutagen 49, 100-109. doi:10.1002/em.20370
Craighead, J. E. and Mossman, B. T. (1982). The pathogenesis of asbestos-associated diseases. N Engl J Med 306, 1446-1455. doi:10.1056/nejm198206173062403
Davis, J. M., Addison, J., Bolton, R. E. et al. (1986). The pathogenicity of long versus short fibre samples of amosite asbestos administered to rats by inhalation and intraperitoneal injection. Br J Exp Pathol 67, 415-430.
Dekkers, S., Oomen, A. G., Bleeker, E. A. J. et al. (2016). Towards a nanospecific approach for risk assessment. Regul Toxicol Pharmacol 80, 46-59. doi:10.1016/j.yrtph.2016.05.037
Di Cristo, L., Oomen, A. G., Dekkers, S. et al. (2021). Grouping hypotheses and an integrated approach to testing and assessment of nanomaterials following oral ingestion. Nanomaterials 11, 2623. doi:10.3390/nano11102623
Di Cristo, L., Janer, G., Dekkers, S. et al. (2022). Integrated approaches to testing and assessment for grouping nanomaterials following dermal exposure. Nanotoxicology 16, 310-332. doi:10.1080/17435390.2022.2085207
Donaldson, K., Murphy, F. A., Duffin, R. et al. (2010). Asbestos, carbon nanotubes and the pleural mesothelium: A review of the hypothesis regarding the role of long fibre retention in the parietal pleura, inflammation and mesothelioma. Part Fibre Toxicol 7, 5. doi:10.1186/1743-8977-7-5
Duffin, R., Tran, L., Brown, D. et al. (2007). Proinflammogenic effects of low-toxicity and metal nanoparticles in vivo and in vitro: Highlighting the role of particle surface area and surface reactivity. Inhal Toxicol 19, 849-856. doi:10.1080/08958370701479323
EC (2006). Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), establishing a European Chemicals Agency, amending Directive 1999/45/EC and repealing Council Regulation (EEC) no 793/93 and Commission Regulation (EC) no 1488/94 as well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC. OJ L 396. https://eur-lex.europa.eu/eli/reg/2006/1907
EC (2008). Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006. OJ L 353, 1-1355. https://eur-lex.europa.eu/eli/reg/2008/1272
EC (2010). Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. OJ L 276, 33-79. https://eur-lex.europa.eu/eli/dir/2010/63
EC (2018). Commission Regulation (EU) amending Regulation (EC) No 1907/2006 of the European Parliament and of the Council on the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) as regards Annexes I, III,VI, VII, VIII, IX, X, XI, and XII to address nanoforms of substances. OJ L 308, 1-20. https://eur-lex.europa.eu/eli/reg/2018/1881
ECHA – European Chemicals Agency (2008). Guidance on Information Requirements and Chemical Safety Assessment. Chapter R.6: QSARS and grouping of chemicals. Guidance for the implementation of REACH. https://echa.europa.eu/guidance-documents/guidance-on-information-requirements-and-chemical-safety-assessment
ECHA (2017a). Read-Across Assessment Framework (RAAF). doi:10.2823/619212
ECHA (2017b). Appendix R.6-1 for nanomaterials applicable to the guidance on QSARS and grouping of chemicals. In Guidance on Information Requirements and Chemical Safety Assessment. doi:10.2823/273911
Fernández-Cruz, M. L., Hernández-Moreno, D., Catalán, J. et al. (2018). Quality evaluation of human and environmental toxicity studies performed with nanomaterials – The GUIDEnano approach. Environ Sci Nano 5, 381-397. doi:10.1039/C7EN00716G
Fraser, K., Kodali, V., Yanamala, N. et al. (2020). Physicochemical characterization and genotoxicity of the broad class of carbon nanotubes and nanofibers used or produced in U.S. facilities. Part Fibre Toxicol 17, 62. doi:10.1186/s12989-020-00392-w
Grosse, Y., Loomis, D., Guyton, K. Z. et al. (2014). Carcinogenicity of fluoro-edenite, silicon carbide fibres and whiskers, and carbon nanotubes. Lancet Oncol 15, 1427-1428. doi:10.1016/S1470-2045(14)71109-X
Hunt, N. (2021). Guidance on the GRACIOUS Framework for grouping and read-across of nanomaterials and nanoforms (1.0). Zenodo. doi:10.5281/zenodo.5534465
ISO (2017). ISO/TR 19057:2017 Nanotechnologies – Use and application of acellular in vitro tests and methodologies to assess nanomaterial biodurability. https://www.iso.org/standard/63836.html
Jeliazkova, N., Bleeker, E., Cross, R. et al. (2022). How can we justify grouping of nanoforms for hazard assessment? Concepts and tools to quantify similarity. NanoImpact 25, 100366. doi:doi:10.1016/j.impact.2021.100366
Krug, H. F. (2014). Nanosafety research – Are we on the right track? Angew Chem Int Ed Engl 53, 12304-12319. doi:10.1002/anie.201403367
Kuempel, E. D., Geraci, C. L. and Schulte, P. A. (2012). Risk assessment and risk management of nanomaterials in the workplace: Translating research to practice. Ann Occup Hyg 56, 491-505. doi:10.1093/annhyg/mes040
Lamon, L., Aschberger, K., Asturiol, D. et al. (2019). Grouping of nanomaterials to read-across hazard endpoints: A review. Nanotoxicology 13, 100-118. doi:10.1080/17435390.2018.1506060
Landsiedel, R. (2016). Concern-driven integrated approaches for the grouping, testing and assessment of nanomaterials. Environ Pollut 218, 1376-1380. doi:10.1016/j.envpol.2015.10.060
Mech, A., Rasmussen, K., Jantunen, P. et al. (2019). Insights into possibilities for grouping and read-across for nanomaterials in EU chemicals legislation. Nanotoxicology 13, 119-141. doi:10.1080/17435390.2018.1513092
Murphy, F., Dekkers, S., Braakhuis, H. et al. (2021). An integrated approach to testing and assessment of high aspect ratio nanomaterials and its application for grouping based on a common mesothelioma hazard. NanoImpact 22, 100314. doi:10.1016/j.impact.2021.100314
Murphy, F., Jacobsen, N. R., Di Ianni, E. et al. (2022). Grouping MWCNTs based on their similar potential to cause pulmonary hazard after inhalation: a case-study. Part Fibre Toxicol 19, 50. doi:10.1186/s12989-022-00487-6
Nagai, H., Okazaki, Y., Chew, S. H. et al. (2011). Diameter and rigidity of multiwalled carbon nanotubes are critical factors in mesothelial injury and carcinogenesis. Proc Natl Acad Sci U S A 108, E1330-E1338. doi:10.1073/pnas.1110013108
OECD (2016). Grouping and Read-Across for the Hazard Assessment of Manufactured Nanomaterials. Series on the Safety of Manufactured Nanomaterials, No. 76. OECD Publishing, Paris. https://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=env/jm/mono(2016)59&doclanguage=en
OECD (2017a). Guidance on Grouping of Chemicals. Second Edition. OECD Series on Testing and Assessment, No 194. OECD Publishing, Paris. doi:10.1787/9789264274679-en
OECD (2017b). Guidance Document on the Reporting of Defined Approaches to be Used Within Integrated Approaches to Testing and Assessment. OECD Series on Testing and Assessment, No. 255. OECD Publishing, Paris. doi:10.1787/9789264274822-en
OECD (2018). Test No. 451: Carcinogenicity studies. OECD Guidelines for the Testing of Chemicals, Section 4. OECD Publishing, Paris. doi:10.1787/9789264071186-en
Oomen, A. G., Bleeker, E. A., Bos, P. M. et al. (2015). Grouping and read-across approaches for risk assessment of nanomaterials. Int J Environ Res Public Health 12, 13415-13434. doi:10.3390/ijerph121013415
Patlewicz, G., Ball, N., Boogaard, P. J. et al. (2015). Building scientific confidence in the development and evaluation of read-across. Regul Toxicol Pharmacol 72, 117-133. doi:10.1016/j.yrtph.2015.03.015
Poland, C. A., Duffin, R., Kinloch, I. et al. (2008). Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat Nanotechnol 3, 423-428. doi:10.1038/nnano.2008.111
Rittinghausen, S., Hackbarth, A., Creutzenberg, O. et al. (2014). The carcinogenic effect of various multi-walled carbon nanotubes (MWCNTs) after intraperitoneal injection in rats. Part Fibre Toxicol 11, 59. doi:10.1186/s12989-014-0059-z
Selikoff, I. J., Churg, J. and Hammond, E. C. (1964). Asbestos exposure and neoplasia. JAMA 188, 22-26. doi:10.1001/jama.1964.03060270028006
Sellers, K., Deleebeeck, N., Messiaen, M. et al. (2015). Grouping Nanomaterials – A Strategy Towards Grouping and Read-Across.
Stayner, L., Welch, L. S. and Lemen, R. (2013). The worldwide pandemic of asbestos-related diseases. Annu Rev Public Health 34, 205-216. doi:10.1146/annurev-publhealth-031811-124704
Stone, V., Gottardo, S., Bleeker, E. A. J. et al. (2020). A framework for grouping and read-across of nanomaterials- supporting innovation and risk assessment. Nano Today 35, 100941. doi:10.1016/j.nantod.2020.100941
United Nations (2021). Globally Harmonized System of Classification and Labelling of Chemicals (GHS). 9th edition. https://unece.org/sites/default/files/2021-09/GHS_Rev9E_0.pdf
Verdon, R., Stone, V., Murphy, F. et al. (2022). The application of existing genotoxicity methodologies for grouping of nanomaterials: Towards an integrated approach to testing and assessment. Part Fibre Toxicol 19, 32. doi:10.1186/s12989-022-00476-9
Wagner, J. C., Sleggs, C. A. and Marchand, P. (1960). Diffuse pleural mesothelioma and asbestos exposure in the North Western Cape Province. Br J Ind Med 17, 260-271. doi:10.1136/oem.17.4.260