In vitro-based prediction of human plasma concentrations of food-related compounds
Main Article Content
Abstract
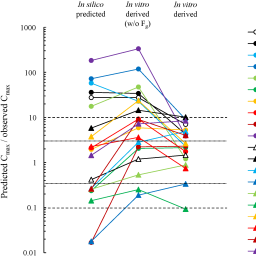
Efforts have been made to replace animal experiments in safety evaluations, including in vitro-based predictions of human internal exposures, such as predicting peak plasma concentration (Cmax) values for xenobiotics and comparing these values with in vitro-based toxicity endpoints. Herein, the authors predicted the Cmax values of food-related compounds in humans based on existing and novel in vitro techniques. In this study, 20 food-related compounds, which have been previously reported in human pharmacokinetic or toxicokinetic studies, were evaluated. Human induced pluripotent stem cell-derived small intestinal epithelial cells (hiPSC-SIEC) and Caco-2 cells, HepaRG cells, equilibrium dialysis of human plasma, and LLC-PK1 cell monolayer were used to assess intestinal absorption and availability, hepatic metabolism, unbound plasma fraction, and secretion and reabsorption in renal tubular cells, respectively. After conversion of these parameters into human kinetic parameters, the plasma concentration profiles of these compounds were predicted using in silico methods, and the obtained Cmax values were found to be between 0.017 and 183 times the reported Cmax values. When the in silico-predicted parameters were modified with in vitro data, the predicted Cmax values came within 0.1-10 times the reported values because the metabolic activities of hiPSC-SIECs, such as uridine 5’-diphospho-glucuronosyl transferase, are more similar to those of human primary enterocytes. Thus, combining in vitro test results with the plasma concentration simulations resulted in more accurate and transparent predictions of Cmax values of food-related compounds than those obtained using in silico-derived predictions alone. This method facilitates accurate safety evaluation without the need for animal experiments.
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.
Articles are distributed under the terms of the Creative Commons Attribution 4.0 International license (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution and reproduction in any medium, provided the original work is appropriately cited (CC-BY). Copyright on any article in ALTEX is retained by the author(s).
Araki, T., Iwazaki, N., Ishiguro, N. et al. (2016). Requirements for human iPS cell-derived hepatocytes as an alternative to primary human hepatocytes for assessing absorption, distribution, metabolism, excretion and toxicity of pharmaceuticals. Fundam Toxicol Sci 3, 89-99. doi:10.2131/fts.3.89
Baltazar, M. T., Cable, S., Carmichael, P. L. et al. (2020). A next-generation risk assessment case study for coumarin in cosmetic products. Toxicol Sci 176, 236-252. doi:10.1093/toxsci/kfaa048
Berellini, G. and Lombardo, F. (2019). An accurate in vitro prediction of human VDss based on the Øie-Tozer equation and primary physicochemical descriptors. 3. Analysis and assessment of predictivity on a large dataset. Drug Metab Dispos 47, 1380-1387. doi:10.1124/dmd.119.088914
Bonn, B., Svanberg, P., Janefeldt, A. et al. (2016). Determination of human hepatocyte intrinsic clearance for slowly metabolized compounds: Comparison of a primary hepatocyte/stromal cell co-culture with plated primary hepatocytes and HepaRG. Drug Metab Dispos 44, 527-533. doi:10.1124/dmd.115.067769
Bury, D., Alexander-White, C., Clewell, H. J. 3rd et al. (2021). New framework for a non-animal approach adequately assures the safety of cosmetic ingredients – A case study on caffeine. Regul Toxicol Pharmacol 123, 104931. doi 10.1016/j.yrtph.2021.104931
Dent, M., Amaral, R. T., Da Silva, P. A. et al. (2018). Principles underpinning the use of new methodologies in the risk assessment of cosmetic ingredients. Comput Toxicol 7, 20-26. doi:10.1016/j.comtox.2018.06.001
EFSA – European Food Safety Authority (2014). Modern methodologies and tools for human hazard assessment of chemicals. EFSA J 12, 3638. doi:10.2903/j.efsa.2014.3638
Fanti, P., Sawaya, B. P., Custer, L. J. et al. (1999). Serum levels and metabolic clearance of the isoflavones genistein and daidzein in hemodialysis patients. J Am Soc Nephrol 10, 864-871. doi:10.1681/ASN.V104864
Gocht, T., Berggren, E., Ahr, H. J. et al. (2015). The SEURAT-1 approach towards animal free human safety assessment. ALTEX 32, 9-24. doi:10.14573/altex.1408041
Hallifax, D., Foster, J. A. and Houston, J. B. (2010). Prediction of human metabolic clearance from in vitro systems: Retrospective analysis and prospective view. Pharm Res 27, 2150-2161. doi:10.1007/s11095-010-0218-3
Kabeya, T., Mima, S., Imakura, Y. et al. (2020). Pharmacokinetic functions of human induced pluripotent stem cell-derived small intestinal epithelial cells. Drug Metab Pharmacokinet 35, 374-382. doi:10.1016/j.dmpk.2020.04.334
Kamiya, Y., Handa, K., Miura, T. et al. (2022). Machine learning prediction of the three main input parameters of a simplified physiologically based pharmacokinetic model subsequently used to generate time-dependent plasma concentration data in humans after oral doses of 212 disparate chemicals. Biol Pharm Bull 45, 124-128. doi:10.1248/bpb.b21-00769
Kikuchi, T., Shigemura, S., Ito, Y. et al. (2022). Determination of human FaFg of polyphenols using allometric scaling. J Toxicol Sci 47, 409-420. doi:10.2131/jts.47.409
Kilford, P. J., Gertz, M., Houston, J. B. et al. (2008). Hepatocellular binding of drugs: Correction for unbound fraction in hepatocyte incubations using microsomal binding or drug lipophilicity data. Drug Metab Dispos 36, 1194-1197. doi:10.1124/dmd.108.020834
Kitaguchi, T., Mizota, T., Ito, M. et al. (2021). Simultaneous evaluation of membrane permeability and UDP-glucuronosyltransferase-mediated metabolism of food-derived compounds using human induced pluripotent stem cell-derived small intestinal epithelial cells. Drug Metab Dispos 50, 17-23. doi:10.1124/dmd.121.000605
Kojima, H. (2019). Use of non-animal test methods in the safety assessment of chemicals. Translat Regulat Sci 1, 66-72. doi:10.33611/trs.1_66
Kunze, A., Huwyler, J., Poller, B. et al. (2014). In vitro-in vivo extrapolation method to predict human renal clearance of drugs. J Pharm Sci 103, 994-1001. doi:10.1002/jps.23851
Lanevskij, K., Didziapetris, R., and Japertas, P. (2010). Trainable QSAR model of plasma protein binding and its application for predicting volume of distribution. American Chemical Society 240th National Meeting, MEDI-402. https://bit.ly/3QGaDcp
Lauschke, V. M., Shafagh, R. Z., Hendriks, D. F. G. et al. (2019). 3D primary hepatocyte culture systems for analyses of liver diseases, drug metabolism, and toxicity: Emerging culture paradigms and applications. Biotechnol J 14, e1800347. doi:10.1002/biot.201800347
Lea, T. (2015). Caco-2 cell line. In K. Verhoeckx, P. Cotter, I. Lópz-Expósito et al., The Impact of Food Bioactives on Health. Berlin, Germany: Springer.
Lu, R., Zhou, Y., Ma, J. et al. (2022). Strategies and mechanism in reversing intestinal drug efflux in oral drug delivery. Pharmaceutics 14, 1131. doi:10.3390/pharmaceutics14061131
Luo, G., Johnson, S., Hsueh, M. M. et al. (2010). In silico prediction of biliary excretion of drugs in rats based on physicochemical properties. Drug Metab Dispos 38, 422-430. doi:10.1124/dmd.108.026260
Michiba, K., Maeda, K., Shimomura, O. et al. (2022). Usefulness of human jejunal spheroid-derived differentiated intestinal epithelial cells for the prediction of intestinal drug absorption in humans. Drug Metab Dispos 50, 204-213. doi:10.1124/dmd.121.000796
Murota, K., Shimizu, S., Miyamoto, S. et al. (2002). Unique uptake and transport of isoflavone aglycones by human intestinal caco-2 cells: Comparison of isoflavonoids and flavonoids. J Nutr 132, 1956-1961. doi:10.1093/jn/132.7.1956
Nakamori, F., Naritomi, Y., Hosoya, K. et al. (2012). Quantitative prediction of human intestinal glucuronidation effects on intestinal availability of UDP-glucuronosyltransferase substrates using in vitro data. Drug Metab Dispos 40, 1771-1777. doi:10.1124/dmd.112.045476
Nishimuta, H., Sato, K., Yabuki, M. et al. (2011). Prediction of the intestinal first-pass metabolism of CYP3A and UGT substrates in humans from in vitro data. Drug Metab Pharmacokinet 26, 592-601. doi:10.2133/dmpk.DMPK-11-RG-034
Ohta, S., Kimura, S., Maejima, D. et al. (2022). Report on 2021 international workshop for non-animal approaches in the food sector (Japan): Current status and avenues for further research. ALTEX 40, 350-356. doi:10.14573/altex.2209262
Øie, S. and Tozer, T. N. (1979). Effect of altered plasma protein binding on apparent volume of distribution. J Pharm Sci 68, 1203-1205. doi:10.1002/jps.2600680948
Press, B. and Di Grandi, D. (2008). Permeability for intestinal absorption: Caco-2 assay and related issues. Curr Drug Metab 9, 893-900. doi:10.2174/138920008786485119
Punt, A., Louisse, J., Beekmann, K. et al. (2022). Predictive performance of next generation human physiologically based kinetic (PBK) models based on in vitro and in silico input data. ALTEX 39, 221-234. doi:10.14573/altex.210830
Reynolds, D. P., Lanevskij, K., Japertas, P. et al. (2009). Ionization-specific analysis of human intestinal absorption. J Pharm Sci 98, 4039-4054. doi:10.1002/jps.21730
Sazonovas, A., Japertas, P., and Didziapetris, R. (2010). Estimation of reliability of predictions and model applicability domain evaluation in the analysis of acute toxicity (LD50). SAR QSAR Environ Res 21, 127-148. doi:10.1080/10629360903568671
Sugano, K. (ed.) (2012). Biopharmaceutics Modeling and Simulations: Theory, Practice, Methods, and Applications. John Wiley & Sons, Inc. doi:10.1002/9781118354339
Terasaka, S., Hayashi, A., Nukada, Y. et al. (2022). Investigating the uncertainty of prediction accuracy for the application of physiologically based pharmacokinetic models to animal-free risk assessment of cosmetic ingredients. Regul Toxicol Pharmacol 135, 105262. doi:10.1016/j.yrtph.2022.105262
Uwai, Y., Ozeki, Y., Isaka, T. et al. (2011). Inhibitory effect of caffeic acid on human organic anion transporters hOAT1 and hOAT3: A novel candidate for food-drug interaction. Drug Metab Pharmacokinet 26, 486-493. doi:10.2133/dmpk.dmpk-11-rg-020
Vandecasteele, H. A., Gautier, F., Tourneix, F. et al. (2021). Next generation risk assessment for skin sensitisation: A case study with propyl paraben. Regul Toxicol Pharmacol 123, 104936. doi:10.1016/j.yrtph.2021.104936
Waters, N. J. and Lombardo, F. (2010). Use of the Øie-Tozer model in understanding mechanisms and determinants of drug distribution. Drug Metab Dispos 38, 1159-1165. doi:10.1124/dmd.110.032458
Yu, L. X., and Amidon, G. L. (1999). A compartmental absorption and transit model for estimating oral drug absorption. Int J Pharm 186, 119-125. doi:10.1016/s0378-5173(99)00147-7
Zanelli, U., Caradonna, N. P., Hallifax, D. et al. (2012). Comparison of cryopreserved HepaRG cells with cryopreserved human hepatocytes for prediction of clearance for 26 drugs. Drug Metab Dispos 40, 104-110. doi:10.1124/dmd.111.042309