Incorporating new approach methodologies into regulatory nonclinical pharmaceutical safety assessment
Main Article Content
Abstract
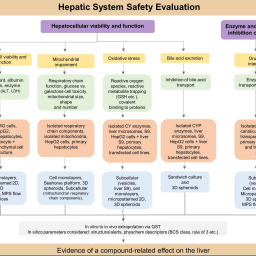
New approach methodologies (NAMs) based on human biology enable the assessment of adverse biological effects of pharmaceuticals and other chemicals. Currently, however, it is unclear how NAMs should be used during drug development to improve human safety evaluation. A series of 5 workshops with 13 international experts (regulators, preclinical scientists, and NAMs developers) was conducted to identify feasible NAMs and to discuss how to exploit them in specific safety assessment contexts. Participants generated four “maps” of how NAMs can be exploited in the safety assessment of the liver, respiratory, cardiovascular, and central nervous systems. Each map shows relevant endpoints measured and tools used (e.g., cells, assays, platforms), and highlights gaps where further development and validation of NAMs remains necessary. Each map addresses the fundamental scientific requirements for the safety assessment of that organ system, providing users with guidance on the selection of appropriate NAMs. In addition to generating the maps, participants offered suggestions for encouraging greater NAM adoption within drug development and their inclusion in regulatory guidelines. A specific recommendation was that pharmaceutical companies should be more transparent about how they use NAMs in-house. As well as giving guidance for the four organ systems, the maps provide a template that could be used for additional organ safety testing contexts. Moreover, their conversion to an interactive format would enable users to drill down to the detail necessary to answer specific scientific and regulatory questions.
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.
Articles are distributed under the terms of the Creative Commons Attribution 4.0 International license (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution and reproduction in any medium, provided the original work is appropriately cited (CC-BY). Copyright on any article in ALTEX is retained by the author(s).
Andersen, M., McMullen, P., Phillips, M. et al. (2019). Developing context appropriate toxicity testing approaches using new alternative methods (NAMs). ALTEX 36, 523-534. doi:10.14573/altex.1906261
Avila, A. M., Bebenek, I., Bonzo, J. A. et al. (2020). An FDA/CDER perspective on nonclinical testing strategies: Classical toxicology approaches and new approach methodologies (NAMs). Regul Toxicol Pharmacol 114, 104662. doi:10.1016/j.yrtph.2020.104662
Bai, J. P. F., Earp, J. C., Strauss, D. G. et al. (2020). A perspective on quantitative systems pharmacology applications to clinical drug development. CPT Pharmacometrics Syst Pharmacol 9, 675-677. doi:10.1002/psp4.12567
Bailey, J., Thew, M. and Balls, M. (2015). Predicting human drug toxicity and safety via animal tests: Can any one species predict drug toxicity in any other, and do monkeys help? Altern Lab Anim 43, 393-403. doi:10.1177/026119291504300607
Balogh Sivars, K., Sivars, U., Hornberg, E. et al. (2018). A 3D human airway model enables prediction of respiratory toxicity of inhaled drugs in vitro. Toxicol Sci 162, 301-308. doi:10.1093/toxsci/kfx255
Bartfeld, S. and Clevers, H. (2017). Stem cell-derived organoids and their application for medical research and patient treatment. J Mol Biol 95, 729-738. doi:10.1007/s00109-017-1531-7
Baudy, A. R., Otieno, M. A., Hewitt, P. et al. (2020). Liver microphysiological systems development guidelines for safety risk assessment in the pharmaceutical industry. Lab Chip 20, 215-225. doi:10.1039/C9LC00768G
Bowes, J., Brown, A. J., Hamon, J. et al. (2012). Reducing safety-related drug attrition: The use of in vitro pharmacological profiling. Nat Rev Drug Discov 11, 909-922. doi:10.1038/nrd3845
Bracher, M., Pilkington, G. J., Hanemann, C. O. et al. (2020). A systematic approach to review of in vitro methods in brain tumour research (SAToRI-BTR): Development of a preliminary checklist for evaluating quality and human relevance. Front Bioeng Biotechnol 8, 1-17. doi:10.3389/fbioe.2020.00936
Burgdorf, T., Piersma, A. H., Landsiedel, R. et al. (2019). Workshop on the validation and regulatory acceptance of innovative 3R approaches in regulatory toxicology – Evolution versus revolution. Toxicol In Vitro 9, 1-11. doi:10.1016/j.tiv.2019.03.039
Busquet, F., Hartung, T., Pallocca, G. et al. (2020). Harnessing the power of novel animal-free test methods for the development of COVID-19 drugs and vaccines. Arch Toxicol 94, 2263-2272. doi:10.1007/s00204-020-02787-2
Butler, L. D., Guzzie-Peck, P., Hartke, J. et al. (2017). Current nonclinical testing paradigms in support of safe clinical trials: An IQ Consortium DruSafe perspective. Regul Toxicol Pharmacol 87, Suppl 3, S1-S15. doi:10.1016/j.yrtph.2017.05.009
Chioccioli, M., Feriani, L., Kotar, J. et al. (2019). Phenotyping ciliary dynamics and coordination in response to CFTR-modulators in cystic fibrosis respiratory epithelial cells. Nat Commun 10, 1763. doi:10.1038/s41467-019-09798-3
Choudhury, Y., Toh, Y. C., Xing, J. et al. (2017). Patient-specific hepatocyte-like cells derived from induced pluripotent stem cells model pazopanib-mediated hepatotoxicity. Sci Rep 7, 41238. doi:10.1038/srep41238
Clippinger, A. J., Allen, D., Jarabek, A. M. et al. (2018). Alternative approaches for acute inhalation toxicity testing to address global regulatory and non-regulatory data requirements: An international workshop report. Toxicol In Vitro 48, 53-70. doi:10.1016/j.tiv.2017.12.011
Cook, D., Brown, D., Alexander, R. et al. (2014). Lessons learned from the fate of AstraZeneca’s drug pipeline: A five-dimensional framework. Nat Rev Drug Discov 13, 419-431. doi:10.1038/nrd4309
Corley, R. A., Kuprat, A. P., Suffield, S. R. et al. (2021). New approach methodology for assessing inhalation risks of a contact respiratory cytotoxicant: Computational fluid dynamics-based aerosol dosimetry modeling for cross-species and in vitro comparisons. Toxicol Sci 182, 243-259. doi:10.1093/toxsci/kfab062
Dirven, H., Vist, G. E., Bandhakavi, S. et al. (2021). Performance of preclinical models in predicting drug-induced liver injury in humans: A systematic review. Sci Rep 11, 6403. doi:10.1038/s41598-021-85708-2
EMA – European Medicines Agency (2018). Reporting of physiologically based pharmacokinetic (PBPK) modelling and simulation. https://bit.ly/3BrHc4O
EMA (2021). Mandate, Objectives and Rules of Procedure of the Scientific Advice Working Party (SAWP). https://bit.ly/3M4IhVb
Ewart, L., Apostolou, A., Briggs, S. A. et al. (2022). Performance assessment and economic analysis of a human Liver-Chip for predictive toxicology. Commun Med 2, 154. doi:10.1038/s43856-022-00209-1
Fernandez-Checa, J. C., Bagnaninchi, P., Ye, H. et al. (2021). Advanced preclinical models for evaluation of drug-induced liver injury – Consensus statement by the European drug-induced liver injury network [PRO-EURO-DILI-NET]. J Hepatol 75, 935-959. doi:10.1016/j.jhep.2021.06.021
Ferrari, E. and Rasponi, M. (2021). Liver-heart on chip models for drug safety. APL Bioeng 5, 031505. doi:10.1063/5.0048986
Fontana, R. J. (2008). Acute liver failure due to drugs. Semin Liver Dis 28, 175-187. doi:10.1055/s-2008-107311747
Fowler, S., Chen, W. L. K., Duignan, D. B. et al. (2020). Microphysiological systems for ADME-related applications: Current status and recommendations for system development and characterization. Lab Chip 20, 446-467. doi:10.1039/C9LC00857H
Franzen, N., van Harten, W. H., Retèl, V. P. et al. (2019). Impact of organ-on-a-chip technology on pharmaceutical R&D costs. Drug Discov Today 24, 1720-1724. doi:10.1016/j.drudis.2019.06.003
Gocke, E., Müller, L., Guzzie, P. J. et al. (2000). Considerations on photochemical genotoxicity: Report of the international workshop on genotoxicity test procedures working group. Environ Mol Metagen 35, 173-184. doi:10.1002/(sici)1098-2280(2000)35:3<173::aid-em4>3.0.co;2-e
Guo, Y. and Pu, W. T. (2020). Cardiomyocyte maturation. Circ Res 126, 1086-1106. doi:10.1161/circresaha.119.315862
Hardy, A., Benford, D., Halldorsson, T. et al. (2017). Update: Use of the benchmark dose approach in risk assessment. EFSA J 15, e04658. doi:10.2903/j.efsa.2017.4658
Hill, E. J., Jiménez-González, C., Tarczyluk, M. et al. (2012). NT2 derived neuronal and astrocytic network signalling. PLoS One 7, e36098. doi:10.1371/journal.pone.0036098
ICCVAM – Interagency Coordinating Committee on the Validation of Alternative Methods. (2018). A Strategic Roadmap for Establishing New Approaches to Evaluate the Safety of Chemicals and Medical Products in the United States. https://ntp.niehs.nih.gov/iccvam/docs/roadmap/iccvam_strategicroadmap_january2018_document_508.pdf
ICH – International Conference on Harmonisation (2000). Guideline S7A Safety Pharmacology Studies for Human Pharmaceuticals. https://database.ich.org/sites/default/files/S7A_Guideline.pdf
ICH (2009). Guideline M3(R2) on Nonclinical Safety Studies for the Conduct of Human Clinical Trials and Marketing Authorisation for Pharmaceuticals Step 5. https://database.ich.org/sites/default/files/M3_R2__Guideline.pdf
ICH (2018). Final Concept Paper on ICH S7B and E14 Q&A; 15 November. https://database.ich.org/sites/default/files/E14S7B_IWG_Concept_Paper.pdf
IPCS and IOMC – International Programme on Chemical Safety and Inter-Organization Programme for the Sound Management of Chemicals (2010). Characterization and application of physiologically based pharmacokinetic models in risk assessment. World Health Organization. https://apps.who.int/iris/handle/10665/44495
Kanda, Y., Yamazaki, D., Osada, T. et al. (2018). Development of torsadogenic risk assessment using human induced pluripotent stem cell-derived cardiomyocytes: Japan iPS cardiac safety assessment (JiCSA) update. J Pharmacol Sci 138, 233-239. doi:10.1016/j.jphs.2018.10.010
Kennedy, R., Kuvshinov, D., Sdrolia, A. et al. (2019). A patient tumour-on-a-chip system for personalised investigation of radiotherapy based treatment regimens. Sci Rep 9, 6327. doi:10.1038/s41598-019-42745-2
Kuprat, A. P., Jalali, M., Jan, T. et al. (2021). Efficient bi-directional coupling of 3D computational fluid-particle dynamics and 1D multiple path particle dosimetry lung models for multiscale modeling of aerosol dosimetry. J Aerosol Sci 151, 105647. doi:10.1016/j.jaerosci.2020.105647
LASA and NC3Rs (2009). Guidance on Dose Level Selection for Regulatory General Toxicology Studies for Pharmaceuticals. http://www.lasa.co.uk/PDF/LASA-NC3RsDoseLevelSelection.pdf
Lauschke, V. M., Vorrink, S. U., Moro, S. M. L. et al. (2016a). Massive rearrangements of cellular microRNA signatures are key drivers of hepatocyte dedifferentiation. Hepatology 64, 1743-1756. doi:10.1002/hep.28780
Lauschke, V. M., Hendriks, D. F. G., Bell, C. C. et al. (2016b). Novel 3D culture systems for studies of human liver function and assessments of the hepatotoxicity of drugs and drug candidates. Chem Res Toxicol 29, 1936-1955. doi:10.1021/acs.chemrestox.6b00150
Lauschke, V. M., Shafagh, R. Z., Hendriks, D. F. G. et al. (2019). 3D primary hepatocyte culture systems for analyses of liver diseases, drug metabolism, and toxicity: Emerging culture paradigms and applications. Biotechnol J 14, 1800347. doi:10.1002/biot.201800347
Lemme, M., Ulmer, B. M., Lemoine, M. D. et al. (2018). Atrial-like engineered heart tissue: An in vitro model of the human atrium. Stem Cell Rep 11, 1378-1390. doi:10.1016/j.stemcr.2018.10.008
Li, W., Luo, X., Ulbricht, Y. et al. (2019). Establishment of an automated patch-clamp platform for electrophysiological and pharmacological evaluation of hiPSC-CMs. Stem Cell Rep 41, 101662. doi:10.1016/j.scr.2019.101662
Lin, C. and Khetani, S. R. (2016). Advances in engineered liver models for investigating drug-induced liver injury. Biomed Res Int 2016, 1829148. doi:10.1155/2016/1829148
Liu, L., Yu, L., Li, Z. et al. (2021). Patient-derived organoid (PDO) platforms to facilitate clinical decision making. J Transl Med 19, 40. doi:10.1186/s12967-020-02677-2
Morgan, P., Brown, D. G., Lennard, S. et al. (2018). Impact of a five-dimensional framework on R&D productivity at AstraZeneca. Nat Rev Drug Discov 17, 167-181. doi:10.1038/nrd.2017.244
Nantasanti, S., de Bruin, A., Rothuizen, J. et al. (2016). Concise review: Organoids are a powerful tool for the study of liver disease and personalized treatment design in humans and animals. Stem Cells Transl Med 5, 325-330. doi:10.5966/sctm.2015-0152
NASEM – National Academies of Sciences, Engineering, and Medicine (2021). Microphysiological Systems: Bridging Human and Animal Research – A Workshop. https://www.nationalacademies.org/event/01-19-2021/microphysiological-systems-bridging-human-and-animal-research-a-workshop
Ouchi, R., Togo, S., Kimura, M. et al. (2019). Modeling steatohepatitis in humans with pluripotent stem cell-derived organoids. Cell Metab 30, 374-384.e6. doi:10.1016/j.cmet.2019.05.007
Pamies, D., Leist, M., Coecke, S. et al. (2022). Guidance document on Good Cell and Tissue Culture Practice 2.0 (GCCP 2.0). ALTEX 39, 30-70. doi:10.14573/altex.2111011
Parish, S. T., Aschner, M., Casey, W. et al. (2020). An evaluation framework for new approach methodologies (NAMs) for human health safety assessment. Regul Toxicol Pharmacol 112, 104592. doi:10.1016/j.yrtph.2020.104592
Patterson, E. A., Whelan, M. P. and Worth, A. P. (2021). The role of validation in establishing the scientific credibility of predictive toxicology approaches intended for regulatory application. Comput Toxicol 17, 100144. doi:10.1016/j.comtox.2020.100144
Peterson, N. C., Mahalingaiah, P. K., Fullerton, A. et al. (2020). Application of microphysiological systems in biopharmaceutical research and development. Lab Chip 20, 697-708. doi:10.1039/C9LC00962K
Piersma, A. H., Burgdorf, T., Louekari, K. et al. (2018). Workshop on acceleration of the validation and regulatory acceptance of alternative methods and implementation of testing strategies. Toxicol In Vitro 50, 62-74. doi:10.1016/j.tiv.2018.02.018
Pointon, A., Harmer, A. R., Dale, I. L. et al. (2015). Assessment of cardiomyocyte contraction in human-induced pluripotent stem cell-derived cardiomyocytes. Toxicol Sci 144, 227-237. doi:10.1093/toxsci/kfu312
Pointon, A., Maher, J., Davis, M. et al. (2021). Cardiovascular microphysiological systems (CVMPS) for safety studies – A pharma perspective. Lab Chip 21, 458-472. doi:10.1039/D0LC01040E
Primavessy, D., Metz, J., Schnur, S. et al. (2021). Pulmonary in vitro instruments for the replacement of animal experiments. Eur J Pharm Biopharm 168, 62-75. doi:10.1016/j.ejpb.2021.08.005
Rubiano, A., Indapurkar, A., Yokosawa, R. et al. (2021). Characterizing the reproducibility in using a liver microphysiological system for assaying drug toxicity, metabolism, and accumulation. Clin Transl Sci 14, 1049-1061. doi:10.1111/cts.12969
Sadler, R. C., Prime, D., Burnell, P. K. et al. (2011). Integrated in vitro experimental modelling of inhaled drug delivery: Deposition, dissolution and absorption. J Drug Deliv Sci Technol 21, 331-338. doi:10.1016/S1773-2247(11)50051-6
Smith, B., Rowe, J., Watkins, P. B. et al. (2020). Mechanistic investigations support liver safety of ubrogepant. Toxicol Sci 177, 84-93. doi:10.1093/toxsci/kfaa093
Su, F. S., Lee, C. F., Rajendran, P. et al. (2020). Nasal airflow simulation on healthy child using computational fluid dynamics. Technol Reports Kansai Univ 62. https://www.kansaiuniversityreports.com/article/nasal-airflow-simulation-on-healthy-child-using-computational-fluid-dynamics
Takayama, K., Morisaki, Y., Kuno, S. et al. (2014). Prediction of interindividual differences in hepatic functions and drug sensitivity by using human iPS-derived hepatocytes. Proc Natl Acad Sci 111, 16772-16777. doi:10.1073/pnas.1413481111
Thomas, D., Chancellor, D., Micklus, A. et al. (2021). Clinical Development Success Rates and Contributing Factors 2011-2020. https://go.bio.org/rs/490-EHZ-999/images/ClinicalDevelopmentSuccessRates2011_2020.pdf
Tsega, E. G. (2018). Computational fluid dynamics modeling of respiratory airflow in tracheobronchial airways of infant, child, and adult. Comput Math Methods Med 2018, 9603451. doi:10.1155/2018/9603451
US EPA – US Environmental Protection Agency (2016). Benchmark Dose Software (BMDS). User Manual. https://www.epa.gov/sites/default/files/2015-11/documents/bmds_manual.pdf
US FDA – US Food and Drug Administration (2017). Predictive Toxicology Roadmap. https://www.fda.gov/files/science & research/published/FDA%27s-Predictive-Toxicology-Roadmap.pdf
US FDA (2018). Physiologically Based Pharmacokinetic Analyses – Format and Content. Guidance for Industry. https://www.fda.gov/media/101469/download
US FDA (2019). Liver Toxicity Knowledge Base (LTKB). https://www.fda.gov/science-research/bioinformatics-tools/liver-toxicity-knowledge-base-ltkb
US FDA (2021a). Innovative Science and Technology Approaches for New Drugs (ISTAND) Pilot Program. https://bit.ly/3O5MnyT
US FDA (2021b). Advancing New Alternative Methodologies (NAMs) at FDA. https://www.fda.gov/media/144891/download
US FDA (2021c). Advancing Alternative Methods at FDA. https://www.fda.gov/science-research/about-science-research-fda/advancing-alternative-methods-fda
Varewyck, M. and Verbeke, T. (2017). Software for benchmark dose modelling. EFSA Support Publ 14, 1170E. doi:10.2903/sp.efsa.2017.EN-1170
Wang, X., Wang, L., Dou, W. et al. (2020). Electrical impedance-based contractile stress measurement of human iPSC-Cardiomyocytes. Biosens Bioelectron 166, 112399. doi:10.1016/j.bios.2020.112399
Ware, B. R., Berger, D. R. and Khetani, S. R. (2015). Prediction of drug-induced liver injury in micropatterned co-cultures containing iPSC-derived human hepatocytes. Toxicol Sci 145, 252-262. doi:10.1093/toxsci/kfv048
Watkins, P. B. (2011). Drug safety sciences and the bottleneck in drug development. Clin Pharmacol Ther 89, 788-790. doi:10.1038/clpt.2011.63
Watkins, P. B. (2020). DILIsym: Quantitative systems toxicology impacting drug development. Curr Opin Toxicol 23-24, 67-73. doi:10.1016/j.cotox.2020.06.003
Woehrling, E. K., Parri, H. R., Tse, E. H. Y. et al. (2015). A predictive in vitro model of the impact of drugs with anticholinergic properties on human neuronal and astrocytic systems. PLoS One 10, e0118786. doi:10.1371/journal.pone.0118786
Woodhead, J. L., Brock, W. J., Roth, S. E. et al. (2017). Application of a mechanistic model to evaluate putative mechanisms of tolvaptan drug-induced liver injury and identify patient susceptibility factors. Toxicol Sci 155, 61-74. doi:10.1093/toxsci/kfw193
Yin, S., Xi, R., Wu, A. et al. (2020). Patient-derived tumor-like cell clusters for drug testing in cancer therapy. Sci Transl Med 12, eaaz1723. doi:10.1126/scitranslmed.aaz1723
Zhang, X., Jiang, T., Chen, D. et al. (2020). Three-dimensional liver models: State of the art and their application for hepatotoxicity evaluation. Crit Rev Toxicol 50, 279-309. doi:10.1080/10408444.2020.1756219