Safety assessment of excipients (SAFE) for orally inhaled drug products
Main Article Content
Abstract
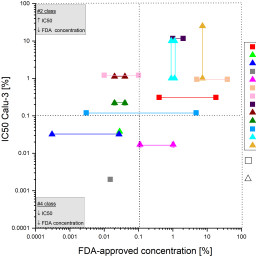
The development of new orally inhaled drug products requires their demonstration of safety, which must be proven in animal experiments. New in vitro methods may replace, or at least reduce, these animal experiments, provided they are able to correctly predict safety or possible toxicity in humans. However, the challenge is to link in vitro data obtained in human cells to human in vivo data. We here present a new approach to the safety assessment of excipients (SAFE) for pulmonary drug delivery. The SAFE model is based on a dose response curve of 23 excipients tested on the human pulmonary epithelial cell lines A549 and Calu-3. The resulting in vitro IC50 values were correlated with the FDA-approved concentrations in pharmaceutical products for either pulmonary (if available) or parenteral administration. Setting a threshold of 0.1% (1 mg/mL) for either value yielded four safety classes and allowed to link IC50 data as measured in human cell cultures in vitro with the concentrations of the same compounds in FDA-approved drug products. The necessary in vitro data for novel excipients can be easily generated, and the SAFE approach allows putting them into context for eventual use in human pulmonary drug products. Excipients that are most likely not safe for use in humans can be excluded early on from further pharmaceutical development. The SAFE approach thus helps to avoid unnecessary animal experiments.
Article Details
Articles are distributed under the terms of the Creative Commons Attribution 4.0 International license (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution and reproduction in any medium, provided the original work is appropriately cited (CC-BY). Copyright on any article in ALTEX is retained by the author(s).